Mechanisms of Meiosis – MOM
Katja WASSMANN
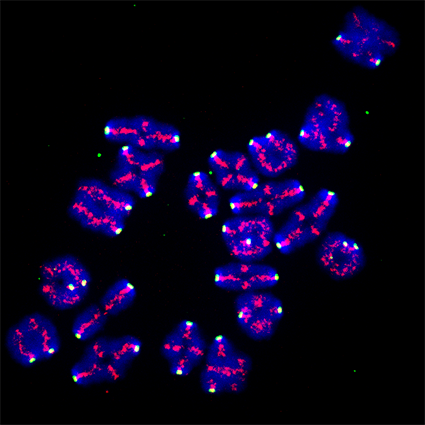
Sexual reproduction requires the generation of haploid gametes through two meiotic divisions, named meiosis I and II. In mammals and especially humans, female gametogenesis is highly error prone, leading to the generation of oocytes harbouring the wrong chromosome content.
Projects in my group aim at dissecting the molecular mechanisms underlying the two meiotic divisions with a focus on oocytes, to understand how errors occur. The central question we study deals with the issue of how meiosis I-specific events are executed only in meiosis I, and those of meiosis II only in meiosis II.
Keywords: Meiosis, Oocytes, Cohesin, Phosphoproteome, Cell cycle, Aneuploidy
+33 (0)1 57 27 81 48 contact @meiosis-mom.bsky.social https://www.researchgate.net/profile/Katja-Wassmann
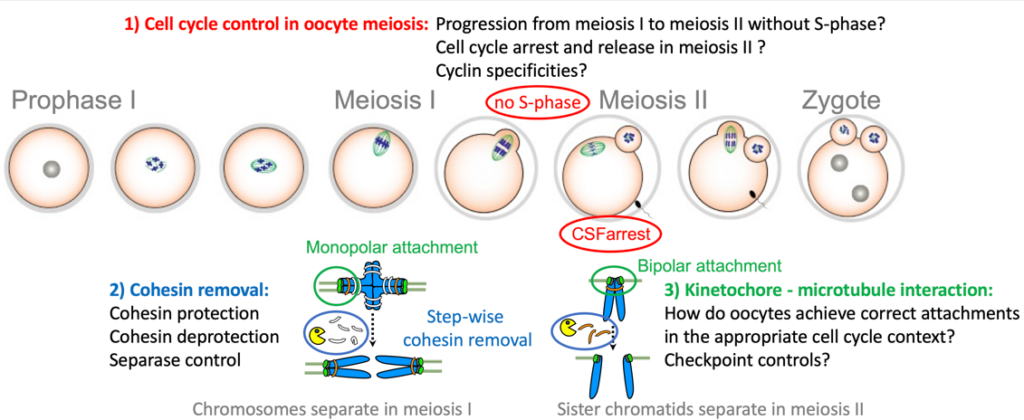
In meiosis, key events need to happen in the correct order, and at the right time, otherwise the generation of healthy gametes is compromised. In our projects we focus on elucidating the underlying regulatory mechanisms of meiosis-specific events with a special focus on oocytes, to gain insights into how these events are put into place in the correct developmental context, e.g. meiosis I or II.Specific features of the meiotic divisions (meiosis I and II):
- Homologous chromosomes (each consisting of two sister chromatids) are paired and segregated in meiosis I, and sister chromatids in meiosis II
- Kinetochores (the attachment sites for the bipolar spindle) of the two paired sister chromatids are co-oriented to the same pole in meiosis I, and bioriented to opposite poles in meiosis II (named mono- and bipolar orientation or attachment, respectively).
- The cohesin complex, which is holding sister chromatids together, is removed in a step-wise manner, from chromosome arms in meiosis I, and the centromere region in meiosis II.
- Transition from meiosis I into meiosis II occurs without intervening S-phase
An additional specificity of vertebrate oocytes is the requirement to implement two cell cycle arrests: One before entry into meiosis I, in prophase I, which lasts decades in humans; a second arrest in metaphase of meiosis II, called CSF-arrest, to await fertilization.
The overarching question of our projects is the following:
How are the two successive meiotic divisions implemented without mixing up meiosis I and meiosis II specific events?
Our research questions:
Model systems we use in the lab: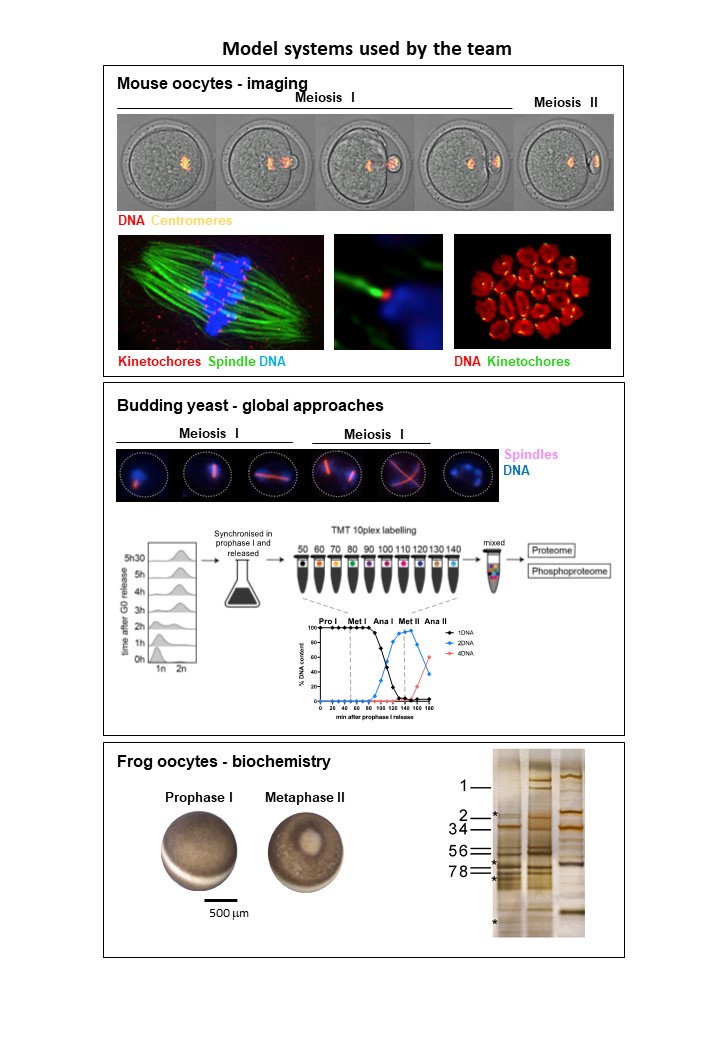
Project 1: Meiotic cell cycle control, Cdk-Cyclin specificities and threshold levels
We think that a global view on mitotic kinase substrate phosphorylation and dephosphorylation, to dissect common principles governing the meiotic divisions, will be key to understand how transition from meiosis I to meiosis II is regulated. Currently we use budding yeast as a model system for this question, to gain insights into this special cell cycle transition in a simple model system, removing an additional layer of complexity that can be expected in oocytes.Additionally, we study Cyclin specificity and Cdk threshold levels for implementing meiosis-specific cell cycle events, using frog and mouse oocytes. We have discovered that at least two M-phase cyclins- Cyclin A2 and B3- occupy specific roles that are not redundant with other cyclins. Both cyclins have functions related to ordering meiosis I and meiosis II-specific events. Our projects aim at identifying specific substrates of these cyclins, and the molecular mechanisms underlying their meiosis-specific roles.
Project 2: Step-wise cohesin removal in mammalian oocytes
Step-wise cohesin removal is brought about by protection of the centromeric cohesin subunit Rec8 from cleavage by the protease Separase at the centromere region in meiosis I, and by removing this protection before sister chromatid segregation in meiosis II. Protection is due to the recruitment of Sgo2, which brings the phosphatase PP2A to the centromere to maintain Rec8 there in a dephosphorylated state. However, several open questions are remaining, starting with the question of how Separase activity is controlled to get inhibited and activated twice, and how proteins involved in protection do so in meiosis I, but not meiosis II, and only in the centromere region.
Project 3: Kinetochore attachments and checkpoint controls in mammalian oocytes
Again related to our main question of how meiosis I and meiosis II events are implemented at the correct time, we want to understand how checkpoints can recognize the correct attachments in their proper context, e.g. monopolar attachments to segregate chromosomes in meiosis I, and bipolar attachments in meiosis II to segregate sister chromatids. Additionally we address whether both kinetochores are functional in meiosis I when they are mono-oriented and fused.
Members
 Eulalie BUFFIN, Assistant Professor, WASSMANN LAB+33 (0)1 57 27 80 35, room 322B
Eulalie BUFFIN, Assistant Professor, WASSMANN LAB+33 (0)1 57 27 80 35, room 322B Esther CHANTY, Biology engineer, WASSMANN LAB+33 (0)1 57 27 80 66, room 322B
Esther CHANTY, Biology engineer, WASSMANN LAB+33 (0)1 57 27 80 66, room 322B Damien CLADIERE, Biology engineer, WASSMANN LAB+33 (0)1 57 27 80 66, room 322B
Damien CLADIERE, Biology engineer, WASSMANN LAB+33 (0)1 57 27 80 66, room 322B Cindy GENOUD, Biology engineer, WASSMANN LAB+33 (0)1 57 27 80 66, room 322B
Cindy GENOUD, Biology engineer, WASSMANN LAB+33 (0)1 57 27 80 66, room 322B Elisavet KOSTANTELIA, Intern, WASSMANN LAB
Elisavet KOSTANTELIA, Intern, WASSMANN LAB Tom LEMONNIER, Postdoctoral researcher, WASSMANN LAB
Tom LEMONNIER, Postdoctoral researcher, WASSMANN LAB Emmanuelle MARJAULT, PhD student, WASSMANN LAB+33 (0)1 57 27 80 66, room 322B
Emmanuelle MARJAULT, PhD student, WASSMANN LAB+33 (0)1 57 27 80 66, room 322B Elena PIZZO, Biology engineer, WASSMANN LAB+33 (0)1 57 27 80 66, room 322B
Elena PIZZO, Biology engineer, WASSMANN LAB+33 (0)1 57 27 80 66, room 322B Irem POLAT, PhD student, WASSMANN LAB+33 (0)1 57 27 80 66, room 322B
Irem POLAT, PhD student, WASSMANN LAB+33 (0)1 57 27 80 66, room 322B Christiane SAADE EL CHAHIR BOUTROS, Intern, WASSMANN LAB
Christiane SAADE EL CHAHIR BOUTROS, Intern, WASSMANN LAB
To contact a member of the team by e-mail: name.surname@ijm.fr

Lab members Corresponding authors of the Lab
El Jailani S., Cladière D., Nikalayevich E., Touati SA., Chesnokova V., Melmed S., Buffin E, Wassmann K. Eliminating separase inhibition reveals absence of robust cohesin protection in oocyte metaphase II. EMBO J. (2025) Sep;44(18):5187-5214.
Langeoire A., Kem-Seng A., Cladière D., Wassmann K., Buffin E. Prolonged metaphase II arrest weakens Aurora B/C-dependent error correction in mouse oocytes. Current Biology (2025) May 5;35(9):2019-2031.e4.
Celebic D., Polat I., Legros V., Chevreux G., Wassmann K., Touati SA. Qualitative rather than quantitative phosphoregulation shapes the end of meiosis I in budding yeast. EMBO J. (2024) Apr;43(7):1325-1350.
Bouftas N.*, Schneider L.*, Halder M., Demmig R., Baack M., Cladière D., Walter M., Al Abdallah H., Kleinhempel C., Messaritaki R., Müller J., Passarelli F., Wehrle P., Heim A., Wassmann K.#+, Mayer T.U.# Cyclin-B3 prevents Emi2/Xerp1 from setting up precocious CSF-arrest in oocyte meiosis I, Dev. Cell (2022) 57, 2305-2320 (*co first-authors, #co-corresponding authors, +lead author)
Nikalayevich E., El Jailani S., Dupré A., Cladière D., Gryaznova Y., Fosse C., Touati S.A., Buffin E., Wassmann K. Aurora B/C-dependent phosphorylation promotes Rec8 cleavage in mammalian oocytes Current Biology (2022) 32:2281-2290 e4
Gryaznova Y., Keating L.*, Touati S.A.*, Caldière D., El Yakoubi W., Buffin E., Wassmann K. Kinetochore individualization in meiosis I is required for centromeric cohesin removal in meiosis II, EMBO J. (2021) 40, e106797 (*co second-authors)
Mehmet E. Karasu*, Bouftas N.*, Keeney S., Wassmann K. Cyclin B3 promotes anaphase I onset in oocyte meiosis, J Cell Biol. (2019) 218, 1265-1281 (*co first-authors)
Vallot A., Leontiou I., El Yakoubi W., Cladière D., Bolte S., Buffin E.*, Wassmann K.*+ Tension-induced error correction, and not kinetochore attachment status activates the SAC in an Aurora-B/C-dependent manner in oocytes, Current Biology (2018) 28, 130-139 (*co-corresponding authors, + lead author)
El Yakoubi W., Buffin E., Cladière D, Gryaznova Y., Berenguer I., Touati S.A., Gómez R., Suja J.A.,van Deursen J.M., Wassmann, K. Mps1 kinase-dependent Sgo2 centromere localization mediates cohesin protection in mouse oocyte meiosis I, Nature Communications (2017) 8:694.
Touati S., Buffin E., Cladière D., Rachez C., Hached K., van Deursen J.M., Wassmann K. Mouse oocytes depend on BubR1 for proper chromosome segregation but not prophase I arrest, Nature Communications (2015) 6:6946
Publications
Theses defended:
- Safia El Jailani: 2021 – 2025

- Dunja Čelebić: 2020 – 2023

- Antoine Langeoire-Cassalta: 2019 – 2023

- Leonor Keating: 2017 – 2021
- Nora Bouftas: 2015 – 2019
- Antoine Vallot: 2014 – 2017
- Sandra Touati: 2011 – 2014
- Khaled Hached: 2006 – 2010
Theses in progress:
- Irem Polat: 2023 –
- Emmanuelle Marjault: 2024 –
- Esther Chanty: 2025 –
- Thomas Mayer, University of Konstanz, Germany
- Evelyn Houliston, Laboratoire Océanologique de Villefranche-sur-Mer, France
- ANR JCJC 2022 – 2025
ANR PRC 2023 –
ANR PRC 2024 –
ARC 2023 – 2024
04/07/2023 – Katja Wassmann, election as a member of the EMBO community
Lab members Corresponding authors of the Lab
El Jailani S., Cladière D., Nikalayevich E., Touati SA., Chesnokova V., Melmed S., Buffin E, Wassmann K. Eliminating separase inhibition reveals absence of robust cohesin protection in oocyte metaphase II. EMBO J. (2025) Sep;44(18):5187-5214.
Langeoire A., Kem-Seng A., Cladière D., Wassmann K., Buffin E. Prolonged metaphase II arrest weakens Aurora B/C-dependent error correction in mouse oocytes. Current Biology (2025) May 5;35(9):2019-2031.e4.
Celebic D., Polat I., Legros V., Chevreux G., Wassmann K., Touati SA. Qualitative rather than quantitative phosphoregulation shapes the end of meiosis I in budding yeast. EMBO J. (2024) Apr;43(7):1325-1350.
Bouftas N.*, Schneider L.*, Halder M., Demmig R., Baack M., Cladière D., Walter M., Al Abdallah H., Kleinhempel C., Messaritaki R., Müller J., Passarelli F., Wehrle P., Heim A., Wassmann K.#+, Mayer T.U.# Cyclin-B3 prevents Emi2/Xerp1 from setting up precocious CSF-arrest in oocyte meiosis I, Dev. Cell (2022) 57, 2305-2320 (*co first-authors, #co-corresponding authors, +lead author)
Nikalayevich E., El Jailani S., Dupré A., Cladière D., Gryaznova Y., Fosse C., Touati S.A., Buffin E., Wassmann K. Aurora B/C-dependent phosphorylation promotes Rec8 cleavage in mammalian oocytes Current Biology (2022) 32:2281-2290 e4
Gryaznova Y., Keating L.*, Touati S.A.*, Caldière D., El Yakoubi W., Buffin E., Wassmann K. Kinetochore individualization in meiosis I is required for centromeric cohesin removal in meiosis II, EMBO J. (2021) 40, e106797 (*co second-authors)
Mehmet E. Karasu*, Bouftas N.*, Keeney S., Wassmann K. Cyclin B3 promotes anaphase I onset in oocyte meiosis, J Cell Biol. (2019) 218, 1265-1281 (*co first-authors)
Vallot A., Leontiou I., El Yakoubi W., Cladière D., Bolte S., Buffin E.*, Wassmann K.*+ Tension-induced error correction, and not kinetochore attachment status activates the SAC in an Aurora-B/C-dependent manner in oocytes, Current Biology (2018) 28, 130-139 (*co-corresponding authors, + lead author)
El Yakoubi W., Buffin E., Cladière D, Gryaznova Y., Berenguer I., Touati S.A., Gómez R., Suja J.A.,van Deursen J.M., Wassmann, K. Mps1 kinase-dependent Sgo2 centromere localization mediates cohesin protection in mouse oocyte meiosis I, Nature Communications (2017) 8:694.
Touati S., Buffin E., Cladière D., Rachez C., Hached K., van Deursen J.M., Wassmann K. Mouse oocytes depend on BubR1 for proper chromosome segregation but not prophase I arrest, Nature Communications (2015) 6:6946




