Sylvain Brun
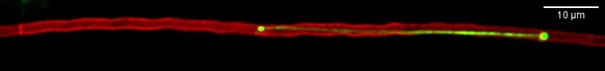
GFP-tagged male nucleus moving from right to left and stretching while passing by septal pore; nuclear membranes are tagged with the mCherry
2003: PhD Thesis: Study of the role of Bcl-2 family proteins in the control of apoptosis in Drosophila melanogaster. LGBC Laboratory, University of Versailles–Saint-Quentin-en-Yvelines, under the co-supervision of Professors B. Mignotte and I. Guénal.
2003–2005: Postdoctoral fellowship: Search for new immune response mutants in Drosophila melanogaster. Host–pathogen interaction team (B. Lemaitre), CGM/I2BC, CNRS, Gif-sur-Yvette.
2005–2007: Postdoctoral fellowship: Study of the Mediator of transcription in Saccharomyces cerevisiae, SBGM/I2BC (M. Werner), CEA, Saclay.
2007–2021: Within the Genetics and Epigenetics of Fungi team (headed by Prof. P. Silar), where I was appointed Maître de Conférences (Associate Professor), I mainly studied the role of the NADPH oxidase complex in the regulation of several aspects of the physiology and life cycle of the filamentous ascomycete fungus Podospora anserina—such as ascospore germination, defense (against other fungi), cellulose penetration, and sexual reproduction. In 2016, I specialized in the study of the cell biology of sexual reproduction in fungi (Neurospora crassa and Podospora anserina), focusing particularly on nuclear movement during this process, during a sabbatical stay at the University of Manchester in Professor Nick Read’s laboratory (Manchester Fungal Infection Group – MFIG). In 2017, I obtained my HDR (Habilitation à Diriger des Recherches) at Université Paris Cité.
Since January 2022, I have joined the Polarity and Morphogenesis team, where I study nuclear movement, deformation, and the cytoskeletal structures underlying these dynamics during sexual reproduction in the model filamentous ascomycete fungus Podospora anserina.
Mechanotransduction of signals, or how mechanical stresses applied to the cell and in particular the nucleus control gene expression, is a rapidly expanding field. This is particularly true for the nuclei of metastatic cells, which deform to migrate through tissues, and an increasing number of pathologies have been found to originate from defects in mechanotransduction or nuclear positioning.
During sexual reproduction in Podospora anserina, a model filamentous ascomycete fungus, male and female nuclei do not fuse immediately after plasmogamy. Instead, the male nuclei migrate along the trichogyne (the female cell), contorting as they pass through the septal pores that separate successive compartments of the trichogyne (Viron et al., 2025; DOI: 10.1101/2025.06.08.658251). During migration, the male nuclei undergo dramatic stretching—up to 12 times their original size—and reach unprecedented speeds of up to 135 µm/min, faster than any nuclear movement previously observed in other organisms.
We recently demonstrated that both the movement and deformation of male nuclei depend on the polarized microtubule network within the trichogynes. To understand these exceptionally rapid movements and to determine how the fungal nucleus tolerates such extreme deformations without rupturing, our studies focus on the microtubule-associated motor proteins and nuclear components—such as the LINC complex—that may regulate these remarkable nuclear movements and deformations.
Co–Principal Investigator
- 2023–2025: IDEX Emergence Université Paris Cité – Project MOVEFORFUN (with S. Dmitrieff, IJM)
- 2021–2023: IDEX Emergence Université Paris Cité – Project NUDE (with F. Bernard, IJM)
- 2014–2016: PHC CEDRE Franco-Lebanese Program (with Z. Kambris, AUB, Lebanon)
Participant
- 2022 & 2023: MITI–CNRS (PI: Y. Sivry)
- 2021–2023: SPE INRAE – Project ARSEN (PI: T.S. Nguyen)
- 2019–2022: IDEX Université Paris Cité – Centre des Politiques de la Terre – Project Meth’InTerE (Co–PIs: P. Grouiez & G. Ruprich-Robert)
Internal: Virginie Courtier-Orgogozo (Evolution and Genetics team); Serge Dmitrieff (Spatial Organization of the Cell team).
External – current:
- Prof. F. Malagnac (I2BC, CNRS–Université Paris-Saclay)
- Prof. L. Peraza-Reyes (UNAM, Mexico)
- D. Tharreau (CIRAD, Montpellier)
- T.S. Nguyen (INRAE BIOGER)
- J.G. Berrin (INRAE, Université Marseille 2)
- Prof. Z. Kambris (American University of Beirut)
External – past:
- Prof. N. Read (University of Manchester)
- Prof. P. Tudzynski (University of Münster)
- R. Debuchy (I2BC, CNRS–Université Paris-Saclay)
PhD:
- Alexander Demoor (2017–2021)
Master 2:
- Zoé Kachaner (2025)
- Marie-Caroline Viron (2024)
- Alexander Demoor (2017)
- Isabelle Lacaze (2012)
- Charlène Girodet (2011)
Master 1:
- L. Mayer (2025)
- Z. Kachaner (2024)
- M-C. Viron (2023)
- T. Bizeau (2022)
- M. Ryckner (2021)
- S. Castro (2019)
- A. Mercier (2015)
- C. Boucher (2015)
Bachelor (L3):
- A. Mercier (2014)
- C. Clairet (2014)
- M. Ducy (2011)
Associate Professor (Maître de Conférences Hors Classe) at Université Paris Cité, teaching genetics, eukaryotic microbiology, and mycology.
Co-head of the Genetics program
Member of the selection committee and co-head of the Microbiology track in the Master 1 BMC (Biology–Molecular and Cellular) program
Co-coordinator of the Genetics courses in L3 Biosciences and L3 Professional MIB programs
Co-coordinator of the Microbiology course in L3 Biosciences and the Fungal Biology course in L2 SDV / L3 SVT programs
Elected representative (category B) to the Board of the Life Sciences Department (UFR Sciences du Vivant), Université Paris Cité
- Articles
- Viron, M-C., Kachaner Z., Grognet P., Lalanne C., Guichet A., Brun S. (2025) BioRiX en révision Preprint at https://doi.org/10.1101/2025.06.08.658251 The fastest movements and the most extreme deformations of the cell nucleus are driven by microtrubules in the model fungus Podospora anserina – auteur pour correspondance
- Guyot, L., Chahine, E., Filippo, E.D., Lalanne, C., Brun, S., Hartmann, F.E., and Giraud, T. (2025) Journal of Evolutionary Biology 38, 1256–1271. Sheltered load in fungal mating-type chromosomes revealed by fitness experiments
- Demoor, A., Lacaze, I., Ferrari R., Lalanne C., Silar, P., and Brun, S. (2023) Microbiology Spectrum. The GUN mutants: new weapons to unravel ascospore germination regulation in the model fungus Podospora anserina -auteur pour correspondance
- Lassagne, A., Brun, S., Malagnac, F., Adreit, H., Milazzo, J., Fournier, E., and Tharreau, D. (2022) Environmental Microbiology. Male fertility in Pyricularia oryzae: Microconidia are spermatia
- Brun, S., Kuo, H.-C., Jeffree, C.E., Thomson, D.D., and Read, N. (2021) Microbiology Spectrum 0, e00335-21. 10.1128/Spectrum.00335-21. Courtship Ritual of Male and Female Nuclei during Fertilization in Neurospora crassa -auteur pour correspondance
- Mercier, A., Clairet, C., Debuchy, R., Morais, D., Silar, P., and Brun, S. (2019) Fungal Genet. Biol. 132, 103257. The mitochondrial translocase of the inner membrane PaTim54 is involved in defense response and longevity in Podospora anserina – auteur pour correspondance
- Demoor, A., Silar, P., and Brun, S. (2019) Fungi Basel Switz. 5. Appressorium: The Breakthrough in Dikarya – auteur pour correspondance
- Ferrari R., Lacaze I., Le Faouder P., Bertrand-Michel J., Oger C., Galano JM., Durand T., Moularat S., Chan Ho Tong L.1, Boucher C.1, Kilani J., Petit Y., Vanparis O., Trannoy C., Brun S., Lalucque H., Malagnac F., Silar P. (2018) Biophys. Acta BBA – Gen. Subj. 1862, 2174–2182. Cyclooxygenases and lipoxygenases are used by the fungus Podospora anserina to repel nematodes
- Xie N., Ruprich-Robert G., Chapeland-Leclerc F., Coppin E, Lalucque H, Brun S., Debuchy R. and Silar.P (2017) Biol. 429,1, 285-305. Inositol-phosphate signaling as mediator for growth and sexual reproduction in Podospora anserina
- Jaber, S., Mercier, A., Knio, K., Brun, S. and Kambris, Z. (2016). Vectors 9, 491. Isolation of fungi from dead arthropods and identification of a new mosquito natural pathogen – co-dernier auteur et auteur pour correspondance
- Lacaze I., Lalucque H., Siegmund U., Silar P., and Brun S. (2015), Molecular Microbiology March; 95(6):1006-1024. Identification of NoxD/Pro41 as the homologue of the p22phox NADPH oxidase subunit in fungi – auteur pour correspondance
- Aït Benkhali J., Coppin E., Brun S., Peraza-Reyes L., Martin T., Dixelius C., Lazar N., Van Tilbeurg H., Debuchy R. (2013), PLoS Genetics, July, 9(7), e1003642. A Network of HMG-box Transcription Factors Regulates Sexual Cycle in the Fungus Podospora anserina
- Coppin E., Berteaux-Lecellier V., Bidard F., Brun S., Ruprich-Robert G., Espagne E., Aït-Benkhali J., Goarin A., Neisser A., Planamente S., Debuchy R., Silar P. (2012), PLoS One, May, 7(5). Systematic deletion of homeobox genes in Podospora anserina uncovers their roles in shaping the fruiting body
- Lalucque H., Malagnac F., Brun S., Kicka S., Silar P. (2012), Genetics, Jun; 191(2), 419-433. A non-mendelian MAPK-generated hereditary unit controlled by a second MAPK pathway in Podospora anserina
- Brun S., Malagnac., Bidard F., Lalucque H., Silar P., (2009), Molecular Microbiology, Oct;74(2):480-496. Functions and regulation of the Nox family in the filamentous fungus Podospora anserina: a new role in cellulose degradation
- Malagnac F., Bidard F., Lalucque H., Brun S., Lambou K., Lebrun MH., Silar P., (2008), Integr. Biol., 1(2):180-181. Convergent evolution of morphogenetic processes in fungi: Role of tetraspanins and NADPH oxidase 2 in plant pathogens and saprobes
- Esnault C., Ghavi-helm Y., Brun S., Soutourina J., Van Berkum N., Boschiero C., Holstege F., Werner M. (2008), Molecular Cell, Aug 8;31(3):337-346. Mediator-dependent recruitment of TFIIH modules in preinititation complex
- Brun S., Kambris Z., Jang IH., Nam HJ., Roméo Y., Takahashi K., Lee WJ., Ueda R., Lemaitre B. (2006), Current Biology, Apr 18;16(8):808-813. A large-scale in vivo RNAi screen identifies five new serine proteases required for Toll activation during the Drosophila immune response
- Brun S., Vidal, S., Spellmann, P., Takahashi, K., Tricoire, H., and Lemaitre, B. (2006), Genes to Cells, Apr;11(4), 397-407. The MAPKKK Mekk1 regulates the expression of turandot stress genes in response to injury in Drosophila
- Jang IH., Chosa N., Kim SH., Nam HJ., Lemaitre B., Ochiai M., Kambris Z., Brun S., Hashimoto C., Ashida M., Brey PT., Lee WJ. (2006), Developmental Cell, Jan;10(1):45-55. A Spatzle-processing enzyme required for Toll signaling activation in Drosophila innate immunity
- Brun S., Rincheval-Arnold A., Colin J., Risler Y., Mignotte B. et Guenal I (2006), Cell Death and Differentiation, Oct;13(10):1752-1762. The myb-related gene stonewall induces both hyperplasia and cell death in Drosophila: rescue of fly lethality by coexpression of apoptosis inducers
- Brun S., Rincheval V., Gaumer S., Mignotte B., and Guénal I. (2002), Oncogene, Sep 19;21(42), 6458-6470. reaper and bax initiate two different apoptotic pathways affecting mitochondria and antagonized by bcl-2 in Drosophila
- Gaumer S., Guénal I., Brun S., Théodore L., and Mignotte B. (2000), Cell Death and Differentiation, Sep;7(9), 804-814. Bcl-2 and Bax mammalian regulators of apoptosis are functional in Drosophila
- Reviews
- Couturier, M., Tangthirasunun, N., Ning, X., Brun, S., Gautier, V., Bennati-Granier, C., Silar, P., and Berrin, J.-G. (2016). Adv. 34, 976–983. Plant biomass degrading ability of the coprophilic ascomycete fungus Podospora anserina
- Gaumer S., Guénal I., Brun S. and Mignotte B. (2002), Médecine Sciences. 18, 875‑880. L’apoptose chez la drosophile : conformisme et originalité
- Book chapter
- Peraza-Reyes, L., Brun S., Grognet P., Malagnac F. The Mycota, Chapter 13 in press Sexual development in Fungi
- Brun S. & Silar P. (2010), Evolutionary Biology – Concepts, Molecular and Morphological Evolution. pp. 317-328. Convergent evolution of morphogenetic processes in fungi
- Mignotte, B., Colin, J., Brun, S., and Guénal, I. (2005). Apoptosis: the fly point of view. In Apoptosis, (India: Kerala), ISBN 81-308-0021-7

