Cell adhesion and mechanics
BENOIT LADOUX & RENÉ MARC MÈGE
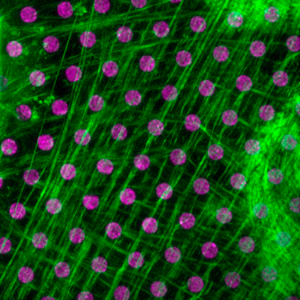
Mechanical constraints and force transmission play an essential role in multicellular living organisms. They are regulating basic biological processes such as morphogenesis, tumor metastasis and tissue repair. Cell adhesions, coupled to the contractile cytoskeleton, are major sites of force transmission in cells. This mechanical coupling which enables cells to sense, signal, and respond to physical changes in the environment, has however been largely understudied. In this context, we are studying the cooperation between adhesion, mechanical and biochemical signaling for the adaptation of living cells to changes in their physical environment at various scales, from single molecules to tissues.
Keywords: Mechanobiology; Mechanosensitivity; epithelial homeostasis; collective migration; microfabrication; biophysics; cell extrusion; tissue mechanics
+33 (0)1 57 27 80 71 / +33 (0)1 57 27 80 67 Contact Benoît LADOUX / René-Marc MEGE @bladoux.bsky.social / @rmmege.bsky.social https://ladoux-mege-lab.cnrs.fr/
At the single cells level
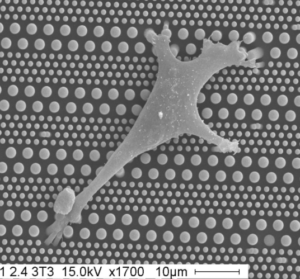
We study force sensing and mechanotransduction at integrin-mediated cell-extracellular matrix and cadherin-mediated cell-cell adhesions. To answer these questions, we have developed single cells and cell doublets models allowing a tight control of cell-matrix and cell-adhesion formation in a physically and mechanically defined microenvironment. Coupled with advanced microscopy, microforce sensor devices and classical cell biology, these approaches allow us to determine the molecular mechanisms that control cell adhesion and cytoskeleton remodeling, as well as cell shape and migration and their adaptation to environment compliance as well as to cytoskeleton visco-elastic properties and cell’s internal tension generated by myosin motors.
At the cell assemblies level
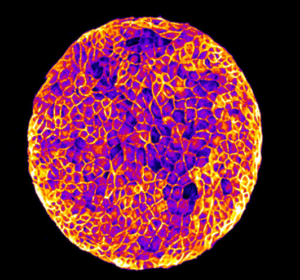
We study the collective behavior of cells in epithelial sheets, in the context of tissue homeostasis and wound healing. To answer these questions, we developing microfabricated tools and biophysical tools to measure and control the mechanical properties and topology of cell’s microenvironment. These tools are combined with molecular approaches, advanced techniques in light microscopy, image analysis and modeling to study the influence of physical properties of the environment on the organization of epithelial layers, collective cell migration, single and collective cell polarization, cell division and cell extrusion. We are characterizing how physical constraints can lead to emergent dynamical and mechanical properties of various epithelial tissues.
At the tissues and organoid level
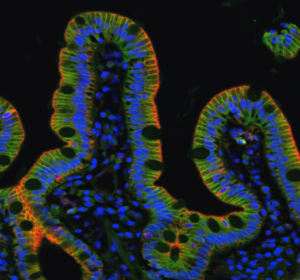
We study how more complex epithelial tissues formed of mixed populations of cells (normal/adhesion deficient, normal/cancer, differentiation/stem cells) facing homogeneous and heterogeneous substrates (extracellular matrix chemistry, rigidity, geometry and topography), regulate homeostasis, segregate and/or auto assemble. Our aims are to determine i) how physical constraints of the microenvironment modulate mechanical properties of epithelial cells and tissues, ii) how they direct a variety of cell behaviors including stem cell proliferation, cell extrusion or delamination, cell migration, differentiation, and polarity, and iii) how they impact on the morphogenesis normal epithelial tissue, as well as the pathological development of intestinal rare diseases. Biomimetic substrates coupled to high resolution imaging and biochemistry are instrumental to reach these goals.
Team leaders
 Benoit LADOUX, Researcher, LADOUX/MEGE LAB+33 (0)1 57 27 80 71, room 142B
Benoit LADOUX, Researcher, LADOUX/MEGE LAB+33 (0)1 57 27 80 71, room 142B Rene-Marc MEGE, Researcher/ImagoSeine coordinator, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B
Rene-Marc MEGE, Researcher/ImagoSeine coordinator, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B
Members
 Lucas ANGER, PhD student, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B
Lucas ANGER, PhD student, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B Gregory ARKOWITZ, PhD student, LADOUX/MEGE LAB+33 (0)1 57 27 80 71, room 142B
Gregory ARKOWITZ, PhD student, LADOUX/MEGE LAB+33 (0)1 57 27 80 71, room 142B Manon ARNAUD, Biology engineer, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B
Manon ARNAUD, Biology engineer, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B Ranjith Kumar CHILUPURI, PhD student, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B
Ranjith Kumar CHILUPURI, PhD student, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B Joseph D ALESSANDRO, Researcher, LADOUX/MEGE LAB+33 (0)1 57 27 80 48, room 142B
Joseph D ALESSANDRO, Researcher, LADOUX/MEGE LAB+33 (0)1 57 27 80 48, room 142B Tien DANG, Biology engineer, LADOUX/MEGE LAB+33 (0)1 57 27 80 68, room 142B
Tien DANG, Biology engineer, LADOUX/MEGE LAB+33 (0)1 57 27 80 68, room 142B Simon DE BECO, Assistant Professor, LADOUX/MEGE LAB+33 (0)1 57 27 80 71, room 142B
Simon DE BECO, Assistant Professor, LADOUX/MEGE LAB+33 (0)1 57 27 80 71, room 142B Olivier DESTRIAN, Postdoctoral researcher, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B
Olivier DESTRIAN, Postdoctoral researcher, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B Robin DURAND, Intern, LADOUX/MEGE LAB
Robin DURAND, Intern, LADOUX/MEGE LAB Marc Antoine FARDIN, Researcher, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B
Marc Antoine FARDIN, Researcher, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B Pan JIANG, Postdoctoral researcher, LADOUX/MEGE LAB+33 (0)1 57 27 80 71, room 142B
Pan JIANG, Postdoctoral researcher, LADOUX/MEGE LAB+33 (0)1 57 27 80 71, room 142B Vanessa PAUL, PhD student, LADOUX/MEGE LAB
Vanessa PAUL, PhD student, LADOUX/MEGE LAB Carine ROSSE, Researcher, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B
Carine ROSSE, Researcher, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B Andreas SCHONIT, PhD student, LADOUX/MEGE LAB+33 (0)1 57 27 80 71, room 142B
Andreas SCHONIT, PhD student, LADOUX/MEGE LAB+33 (0)1 57 27 80 71, room 142B Yuan SHEN, Postdoctoral researcher, LADOUX/MEGE LAB+33 (0)1 57 27 80 68, room 142B
Yuan SHEN, Postdoctoral researcher, LADOUX/MEGE LAB+33 (0)1 57 27 80 68, room 142B Clémence THIANT, PhD student, LADOUX/MEGE LAB+33 (0)1 57 27 80 95, room 142B
Clémence THIANT, PhD student, LADOUX/MEGE LAB+33 (0)1 57 27 80 95, room 142B Hélène VIGNES, Postdoctoral researcher, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B
Hélène VIGNES, Postdoctoral researcher, LADOUX/MEGE LAB+33 (0)1 57 27 80 67, room 142B Fanny WODRASCKA, PhD student, LADOUX/MEGE LAB+33 (0)1 57 27 80 71, room 142B
Fanny WODRASCKA, PhD student, LADOUX/MEGE LAB+33 (0)1 57 27 80 71, room 142B
Pour contacter un membre de l’équipe par mail : prenom.nom@ijm.fr
Balasubramaniam L, Doostmohammadi A, Saw TB, Narayana GHNS, Mueller R, Dang T, Thomas M, Gupta S, Sonam S, Yap AS, Toyama Y, Mège RM, Yeomans JM, Ladoux B. Investigating the nature of active forces in tissues reveals how contractile cells can form extensile monolayers. Nat Mater. 2021 Aug;20(8):1156-1166. doi: 10.1038/s41563-021-00919-2. Epub 2021 Feb 18. Erratum in: Nat Mater. 2021 Mar 9;: PMID: 33603188; PMCID: PMC7611436.
Gaston C, De Beco S, Doss B, Pan M, Gauquelin E, D’Alessandro J, Lim CT, Ladoux B, Delacour D. EpCAM promotes endosomal modulation of the cortical RhoA zone for epithelial organization. Nat Commun. 2021 Apr 13;12(1):2226. doi: 10.1038/s41467-021-22482-9. PMID: 33850145; PMCID: PMC8044225.
Jain S, Cachoux VML, Narayana GHNS, de Beco S, D’Alessandro J, Cellerin V, Chen T, Heuzé ML, Marcq P, Mège RM, Kabla AJ, Lim CT, Ladoux B. The role of single cell mechanical behavior and polarity in driving collective cell migration. Nat Phys. 2020 Jul;16(7):802-809. doi: 10.1038/s41567-020-0875-z. Epub 2020 May 4. PMID: 32641972; PMCID: PMC7343533.
Le AP, Rupprecht JF, Mège RM, Toyama Y, Lim CT, Ladoux B. Adhesion-mediated heterogeneous actin organization governs apoptotic cell extrusion. Nat Commun. 2021 Jan 15;12(1):397. doi: 10.1038/s41467-020-20563-9. PMID: 33452264; PMCID: PMC7810754.
Doss BL, Pan M, Gupta M, Grenci G, Mège RM, Lim CT, Sheetz MP, Voituriez R, Ladoux B. Cell response to substrate rigidity is regulated by active and passive cytoskeletal stress. Proc Natl Acad Sci U S A. 2020 Jun 9;117(23):12817-12825.
doi: 10.1073/pnas.1917555117. Epub 2020 May 22. PMID: 32444491; PMCID: PMC7293595.
Heuzé ML, Sankara Narayana GHN, D’Alessandro J, Cellerin V, Dang T, Williams DS, Van Hest JC, Marcq P, Mège RM, Ladoux B. Myosin II isoforms play distinct roles in <i>adherens</i> junction biogenesis. Elife. 2019 Sep 5;8:e46599. doi: 10.7554/eLife.46599. PMID: 31486768; PMCID: PMC6756789.
Chen T, Callan-Jones A, Fedorov E, Ravasio A, Brugués A, Ong HT, Toyama Y, Low BC, Trepat X, Shemesh T, Voituriez R, Ladoux B. Large-scale curvature sensing by directional actin flow drives cellular migration mode switching. Nat Phys. 2019 Apr;15:393-402. doi: 10.1038/s41567-018-0383-6. Epub 2019 Jan 21. PMID: 30984281; PMCID: PMC6456019.
Seddiki R, Narayana GHNS, Strale PO, Balcioglu HE, Peyret G, Yao M, Le AP, Teck Lim C, Yan J, Ladoux B, Mège RM. Force-dependent binding of vinculin to α-catenin regulates cell-cell contact stability and collective cell behavior.
Mol Biol Cell. 2018 Feb 15;29(4):380-388. doi: 10.1091/mbc.E17-04-0231. Epub 2017 Dec 27. PMID: 29282282; PMCID: PMC6014167.
Saw TB, Doostmohammadi A, Nier V, Kocgozlu L, Thampi S, Toyama Y, Marcq P, Lim CT, Yeomans JM, Ladoux B. Topological defects in epithelia govern cell death and extrusion. Nature. 2017 Apr 12;544(7649):212-216.
Abstract
Salomon J, Gaston C, Magescas J, Duvauchelle B, Canioni D, Sengmanivong L, Mayeux A, Michaux G, Campeotto F, Lemale J, Viala J, Poirier F, Minc N, Schmitz J, Brousse N, Ladoux B, Goulet O, Delacour D. Contractile forces at tricellular contacts modulate epithelial organization and monolayer integrity. Nat Commun. 2017 Jan 13;8:13998.
Abstract
Publications
Preprint
INTERNATIONAL
Alexandre Kabla
Cambridge University, UK
Xavier Trepat
IBEC, Spain
Alpha Yap
University of Queensland, Australia
Julia Yeomans
Oxford University, UK
Michael Sheetz
Pakorn tony Kanchanawong
Lim chwee Teck
Yusuke Tonama
Yan Jie
Gianluca Grenci
Mechanobiology Institute (MBI), Singapore
NATIONAL
France
Raphael Voituriez, Philippe Marcq
Sorbonne Université, Paris
Sylvie Hénon
Laboratoire Matière et Systèmes Complexes, Université de Paris
Philippe Chavrier, Christophe Lamaze, Jacques Prost
Institut Curie, Paris
Olivier Goulet
Hôpital Necker-Enfants Malades, Paris
Yong Chen
Ecole Normale Supérieure, Département de Chimie, Paris
Bénédicte Dalaval
CRBM, Montpellier
Internal
Nicolas Borghi
Mechanotransduction: from Cell Surface to Nucleus
Nicolas Minc
Cellular Spatial Organization
Guillaume Romet-Lemonne & Antoine Jégou
Regulation of Actin Assembly Dynamics
28/10/2025 – Andreas Schoenit is the winner of the Mergier Bourdeix Foundation’s French Breakthroughs in Biology award
22/06/2021 – Le prix “Les Grandes Avancées Françaises en Biologie” attribué à Lakshmi Balasubramaniam par l’Académie des Sciences
22/04/2021 – Benoit Ladoux, lauréat de l’ERC Advanced Grant 2020 !


