Polarity and Morphogenesis
Antoine GUICHET
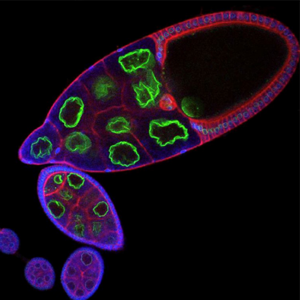
Our research team aims to elucidate the molecular mechanisms that control cell polarity and tissue morphogenesis in relation with the cytoskeleton and in particular with the microtubule network.
Keywords: Cell polarity, morphogenesis, microtubules, asymmetric transport, phospholipids, PAR protein complexes
+33 (0)1 57 27 80 76 Contact @GuichetLab
The understanding of the mechanisms, which orchestrate tissue and organ formation and which control their architecture maintenance, is a fundamental question in biology. Tissue formation and associated homeostasis are coordinated through cellular processes that include cell polarity, cell adhesion and motility. The understanding of these processes is also essential to better understand the molecular mechanisms controlling the development of pathologies such as cancer.
Our research team aims to elucidate the molecular mechanisms that control cell polarity and tissue morphogenesis in relation with the cytoskeleton and in particular with the microtubule network. To do so we are using Drosophila development as model system and we focus our research especially on two axes.
- At a cellular level, by exploring cytoskeleton requirement for the oocyte polarity establishment during oogenesis. We are investigating the molecular mechanisms involved in protein and mRNA asymmetric transport and those required for the asymmetric positioning of the nucleus.
- At a tissue level, by studying cytoskeleton requirement in tissue morphogenesis. We are looking for the molecular processes controlling collective cell migration required for the establishment of the reparatory system during embryogenesis.
We are using both conventional and innovative methodologies by combining genetic, biophysics and cell biology technics. Furthermore, live imaging associated with advanced light microscopy and electron microscopy are the core our experiments.
Nuclear migration in the Drosophila oocyte
Membrane organisation in the Drosophila oocyte
Collective migration of tracheal
cells in the Drosophila embryo
The research topics of the team:
* Identification of mechanisms controlling the asymmetric positioning of the nucleus in the oocyte.
* Identification and characterisation of the different microtubule networks required for the oocyte polarisation.
* Relationship between the lipid domains connected to phosphatidylinositol(4,5) biphosphate and the polarity proteins in the intracellular organisation and the polarized transport.
* Characterisation of the cytoskeleton requirement, microtubules and actin, in the collective cell migration process controlling the formation of the tracheal branches in the embryo.
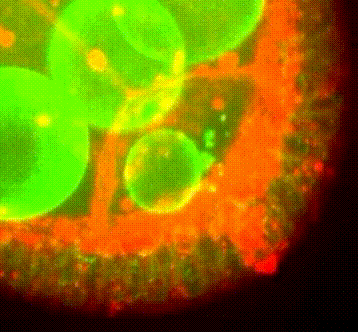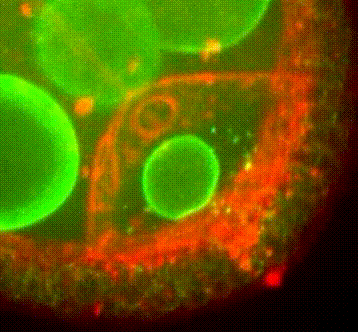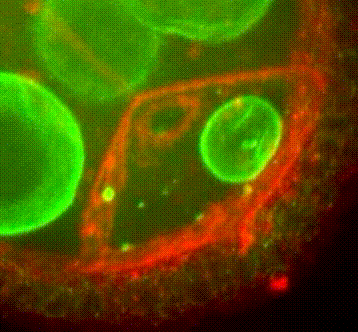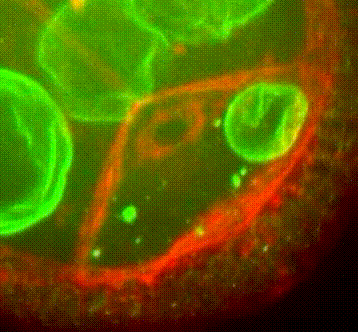
Nuclear migration in the Drosophila oocyte
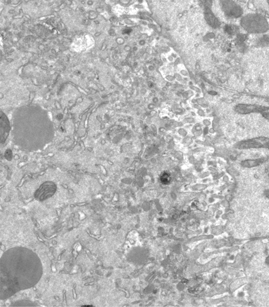
Membrane organisation in the Drosophila oocyte
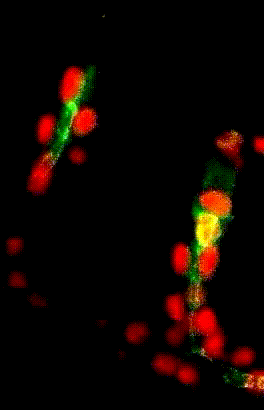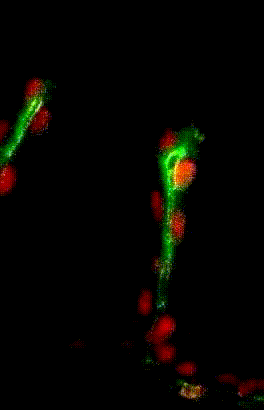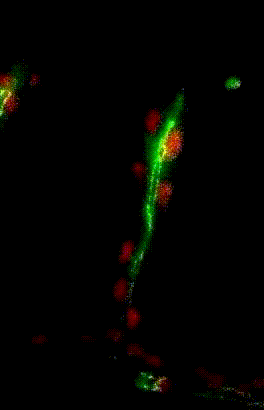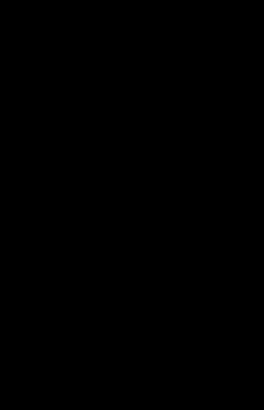
Collective migration of tracheal cells in the Drosophila embryo
Members
 Sandra CARVALHO, PhD student, GUICHET LAB+33 (0)1 57 27 80 76, room 422B
Sandra CARVALHO, PhD student, GUICHET LAB+33 (0)1 57 27 80 76, room 422B Sandra CLARET, Assistant Professor, GUICHET LAB+33 (0)1 57 27 80 77, room 422B
Sandra CLARET, Assistant Professor, GUICHET LAB+33 (0)1 57 27 80 77, room 422B Bleuwenn FERRANDON, PhD student, GUICHET LAB+33 (0)1 57 27 80 76, room 422B
Bleuwenn FERRANDON, PhD student, GUICHET LAB+33 (0)1 57 27 80 76, room 422B Justine FOULIARD, Intern, GUICHET LAB
Justine FOULIARD, Intern, GUICHET LAB Jean-Antoine LEPESANT, Emeritus researcher, GUICHET LAB+33 (0)1 57 27 80 78, room 422B
Jean-Antoine LEPESANT, Emeritus researcher, GUICHET LAB+33 (0)1 57 27 80 78, room 422B Samentha SAHAYAN NEVIL FERNANDO, Intern, GUICHET LAB
Samentha SAHAYAN NEVIL FERNANDO, Intern, GUICHET LAB Wiam SINDABAD, PhD student, GUICHET LAB
Wiam SINDABAD, PhD student, GUICHET LAB
To contact a member of the team by e-mail: name.surname@ijm.fr
The Importance of the Position of the Nucleus in Drosophila Oocyte Development. Lepesant JA, Roland-Gosselin F, Guillemet C, Bernard F, Guichet A. Cells. (2024)
Kinesin-1 promotes centrosome clustering and nuclear migration in the Drosophila oocyte. Development. Loh, M., Bernard, F., Guichet, A. (2023).
Dynein-mediated transport and membrane trafficking control PAR3 polarised distribution. Jouette J, Guichet A, Claret S. eLIFE (2019)
Distinct molecular cues ensure a robust microtubule-dependent nuclear positioning in the Drosophila oocyte.Tissot N, Lepesant JA, Bernard F, Legent K, Bosveld F, Martin C, Faklaris O, Bellaïche Y, Coppey M, Guichet A. Nature Communication. (2017)
Microtubule-dependent apical restriction of recycling endosomes sustains adherens junctions during morphogenesis of the Drosophila tracheal system. Le Droguen PM, Claret S, Guichet A, Brodu V. Development. (2015)
PI(4,5)P2 produced by the PI4P5K Skittles controls the apical domain size by tethering PAR-3 in Drosophila epithelial cells. Claret S, Benoit B, Richard-Ferrec G, Guichet A, Current Biology. (2014)
A developmentally regulated two-step process generates a non-centrosomal microtubule network. Brodu V, Baffet A, Le Droguen PM, Casanova J, Guichet A. Developmental Cell . (2010)
PIP5K-dependent production of PIP2 sustains microtubule organization to establish polarized transport in the Drosophila oocyte. Gervais L, Claret S, Januschke J, Roth S, Guichet A. Development (2008).
The Centrosome Nucleus complex directs the formation of two orthogonal microtubule polarized transport in the Drosophila oocyte Januschke J, Gervais L, Gillet, L., Keryer G, Bornen M, Guichet A. Development. (2006).
Polar transport in the Drosophila oocyte requires Dynein and Kinesin I cooperation, Januschke J, Gervais L, Dass S, Kaltschmidt J, Lopez-Schier H, St. Johnston D, Brand A, Roth S and Guichet A. Current Biology (2002).
Publications
- Maëlys Loh (2022) : Asymétries moléculaires contrôlant le positionnement du noyau dans l’ovocyte de drosophila melanogaster
- Julie Jouette (2017) : Phosphoinositides et contrôle de la polarité cellulaire : régulations croisées entre la PIP5K Skittles et les protéines de polarité PAR1 et PAR3.
- Nicolas Tissot (2015) : Relation croisée entre le positionnement du noyau et l’organisation des microtubules dans la polarisation de l’ovocyte chez la drosophile : approche par microscopie optique ex-vivo et photomanipulation
- Pierre-Marie Le Droguen (2013) : Rôle du réseau de microtubules lors de la morphogénèse du système trachéal dans l’embryon de drosophile
- Alexandre Baffet (2010) : Organisation des microtubules et polarité cellulaire chez la Drosophile
- Julien Compagnon (2008) : Etude du trafic vésiculaire au cours de l’ovogenèse chez la Drosophile
- Louis Gervais (2006): Etude des relations entre la dynamique du réseau de microtubules et le transport polarisé dans l’ovocyte.
- Jens Januschke (2005) : mRNA localization in the Drosophila oocyte
- Dr. Jordi Casanova, Institut de Ciencies de Materials de Barcelona (CSIC), Barcelona, Spain
- Pr. Alexander Ludwig, Nanyang Technological University, Singapore
- Dr. Vladimir Gelfand, Northwestern University, Chicago, USA
- Dr Régis Giet, Institut de Génétique et Développement de Rennes, Rennes
- Dr Paul Conduit, Institut Jacques Monod, Paris
- Nuclear Deformation in Eukaryotes, Projet Emergence en Recherche, IDEX Université Paris Cité (coordinateurs Fred Bernard et Sylvain Brun)
December 17, 2025: Graduation ceremony at the Institut Jacques Monod for the Master’s degree in Science, Technology, and Health, with a major in Molecular and Cellular Biology, specializing in Cell Biology and Development, for students in the class of 2024-2025.





