Stem Cells, Regeneration and Evolution
Eve GAZAVE
 Our team studies the evolution of stem cells and regeneration in animals. The main biological model studied by the team is the annelid worm, Platynereis dumerilii, an emerging model species for developmental and evolutionary studies. We characterize, with a large variety of molecular, cellular, and genomic tools, the amazing regenerative abilities of this animal, with the general aim to determine, through comparative analyses, whether regeneration in animals relies on conserved mechanisms and genetic programs or not. Using a combination of experimental and in silico approaches, we also aim to understand how stem cells involved in development, growth and regeneration have evolved in animals.
Our team studies the evolution of stem cells and regeneration in animals. The main biological model studied by the team is the annelid worm, Platynereis dumerilii, an emerging model species for developmental and evolutionary studies. We characterize, with a large variety of molecular, cellular, and genomic tools, the amazing regenerative abilities of this animal, with the general aim to determine, through comparative analyses, whether regeneration in animals relies on conserved mechanisms and genetic programs or not. Using a combination of experimental and in silico approaches, we also aim to understand how stem cells involved in development, growth and regeneration have evolved in animals.
Keywords: Regeneration, stem cells, evolution, annelids, epigenetics, comparative genomics, development, germ cells, pluripotency, cell reprogramming, growth
+33 (0)1 57 27 81 01 Contact @stemdevevo.bsky.social https://stemdevevo.wordpress.com/
Stem cells, which are able to both self-renew and produce a differentiated progeny, are crucial players of animal embryonic development. They are also involved in post-embryonic processes such as growth, tissue homeostasis, as well as, in some animals, asexual reproduction and regeneration. Regeneration, the ability to restore lost parts of the body, is a widespread phenomenon in animals (reviewed recently in Bideau et al., 2021). Whilst this ability is very limited in mammalians, many animals (such as sponges, cnidarians, planarians, annelids, and salamanders) are able to regenerate complex structures, limbs for example, and in some cases their whole body from a small piece of tissue. Regeneration is often based on the presence of populations of stem cells, either pluripotent and able to regenerate all the different tissues, or multipotent and with a much more restricted potential. Regeneration can also rely on local cell dedifferentiation processes by which differentiated cells are reprogramed into proliferating progenitor or stem cells.
Our team is interested in understanding the evolution of stem cells and regeneration in animals. Our main model to address these topics is the marine annelid Platynereis dumerilii (Figure 1).
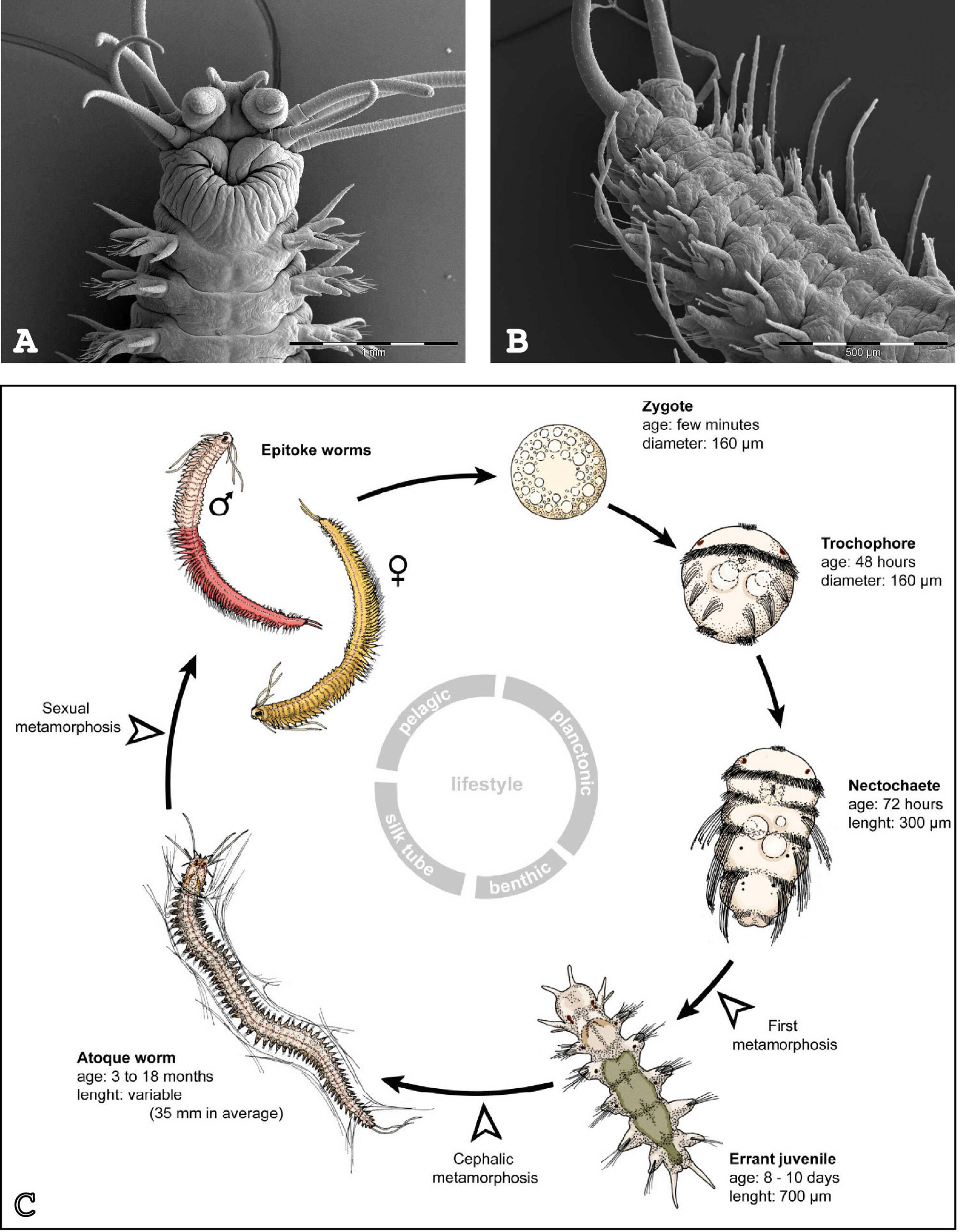
Figure 1 : Platynereis dumerilii and its life cycle. (A) Dorsal view of the head of the adult. (B) Ventral view of the posterior part of the adult. (C) Life cycle. Photographs in (A) and (B) are courtesy of Dr. Nicolas Dray (CNRS). Scheme in C is adapted from Demilly et al. 2013.
Annelid worms belong to lophotrochozoans, the third large evolutionary lineage of the bilaterians, alongside deuterostomes (vertebrates, echinoderms, …) and ecdysozoans (arthropods, nematodes, …). Platynereis has been shown to be extremely valuable for large-scale evolutionary comparisons and is amenable to molecular and functional analyses. After embryonic and larval development, Platynereis worms grow during most of their life, by sequentially adding new segments at their posterior end: a process known as posterior elongation. We showed that this property is linked to the presence of putative stem cells located in a subterminal ‘growth zone’ (GZ) (Gazave et al., 2013). GZ stem cells display a molecular signature (made of the expression of about 20 genes) similar to that of pluripotent stem cells found in other animals and that of primordial germ cells.
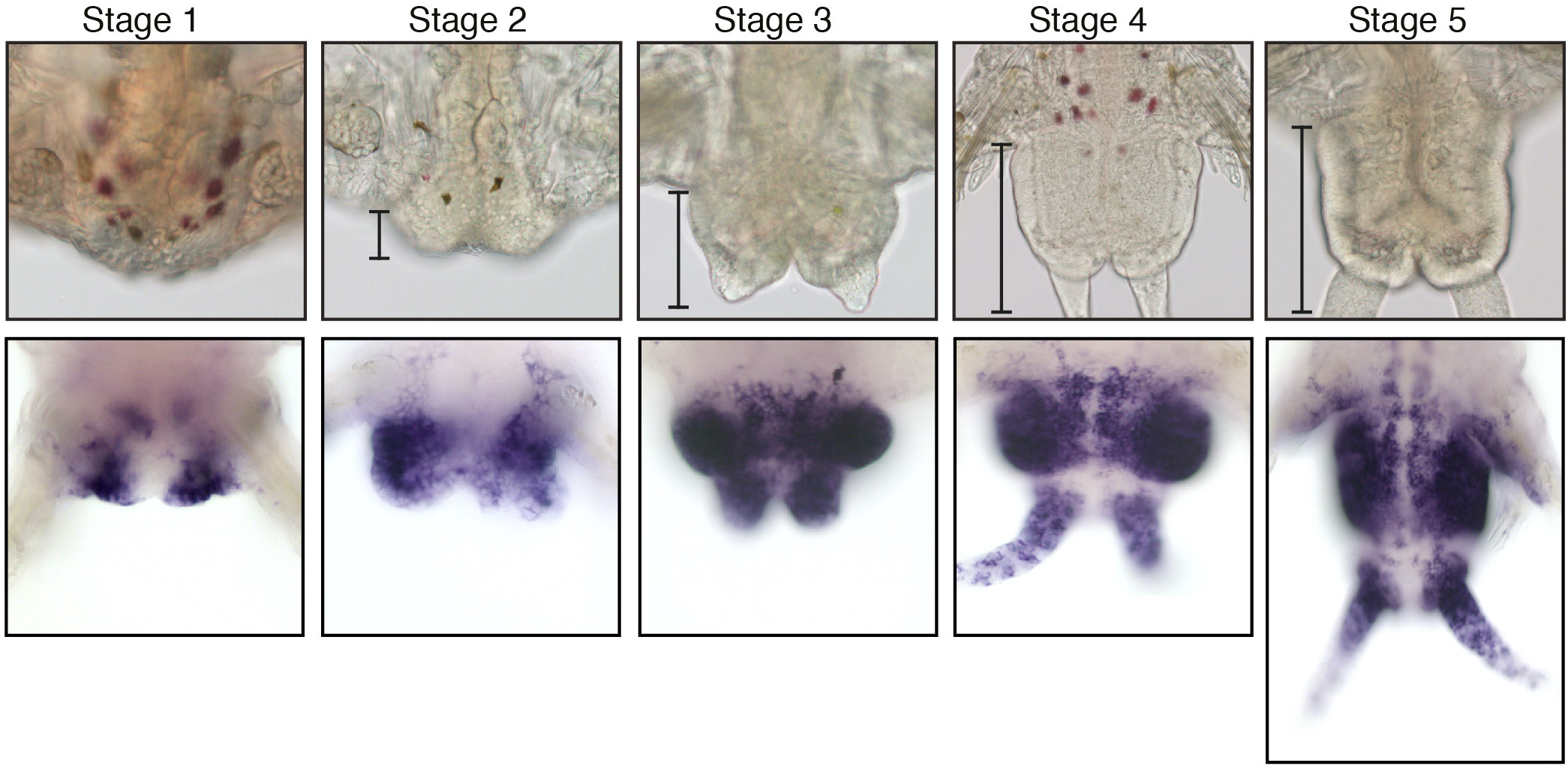
During this growth phase, Platynereis worms also display extensive regeneration abilities. After amputation, Platynereis regenerates the posterior part of its body that includes both the differentiated structures of the posterior-most part of their body and the GZ stem cells whose proliferation allows the replacement of the amputated segments. We performed an in-depth characterization of this regenerative process, which we called posterior regeneration (Figure 2) (Planques et al., 2019). We showed that regeneration is a rapid process that follows a well reproducible path and timeline, going through specific stages. Wound healing is achieved in 1 day and a regeneration blastema forms 1 day later. At this time point, some tissue specification already occurs, and a functional posterior growth zone is re-established as early as 3 days after amputation. We also showed that intense cell proliferation occurs and is strictly required for regeneration to normally proceed.
In addition to its posterior body region, Platynereis worms can also successfully regenerate various types of body outgrowths, in particular their swimming/crawling appendages called parapodia. Platynereis is, in contrast, not able to regenerate its head after an amputation. While the worms can survive for many days after head amputation and a regeneration blastema is formed, the anterior blastema never produces any differentiated head structures (abortive regeneration).
Stem cells, which are able to both self-renew and produce a differentiated progeny, are crucial players of animal embryonic development. They are also involved in post-embryonic processes such as growth, tissue homeostasis, as well as, in some animals, asexual reproduction and regeneration. Regeneration, the ability to restore lost parts of the body, is a widespread phenomenon in animals (reviewed recently in Bideau et al., 2021). Whilst this ability is very limited in mammalians, many animals (such as sponges, cnidarians, planarians, annelids, and salamanders) are able to regenerate complex structures, limbs for example, and in some cases their whole body from a small piece of tissue. Regeneration is often based on the presence of populations of stem cells, either pluripotent and able to regenerate all the different tissues, or multipotent and with a much more restricted potential. Regeneration can also rely on local cell dedifferentiation processes by which differentiated cells are reprogramed into proliferating progenitor or stem cells.
Our team is interested in understanding the evolution of stem cells and regeneration in animals. Our main model to address these topics is the marine annelid Platynereis dumerilii (Figure 1).

Figure 1 : Platynereis dumerilii and its life cycle. (A) Dorsal view of the head of the adult. (B) Ventral view of the posterior part of the adult. (C) Life cycle. Photographs in (A) and (B) are courtesy of Dr. Nicolas Dray (CNRS). Scheme in C is adapted from Demilly et al. 2013.
Annelid worms belong to lophotrochozoans, the third large evolutionary lineage of the bilaterians, alongside deuterostomes (vertebrates, echinoderms, …) and ecdysozoans (arthropods, nematodes, …). Platynereis has been shown to be extremely valuable for large-scale evolutionary comparisons and is amenable to molecular and functional analyses. After embryonic and larval development, Platynereis worms grow during most of their life, by sequentially adding new segments at their posterior end: a process known as posterior elongation. We showed that this property is linked to the presence of putative stem cells located in a subterminal ‘growth zone’ (GZ) (Gazave et al., 2013). GZ stem cells display a molecular signature (made of the expression of about 20 genes) similar to that of pluripotent stem cells found in other animals and that of primordial germ cells.
During this growth phase, Platynereis worms also display extensive regeneration abilities. After amputation, Platynereis regenerates the posterior part of its body that includes both the differentiated structures of the posterior-most part of their body and the GZ stem cells whose proliferation allows the replacement of the amputated segments. We performed an in-depth characterization of this regenerative process, which we called posterior regeneration (Figure 2) (Planques et al., 2019). We showed that regeneration is a rapid process that follows a well reproducible path and timeline, going through specific stages. Wound healing is achieved in 1 day and a regeneration blastema forms 1 day later. At this time point, some tissue specification already occurs, and a functional posterior growth zone is re-established as early as 3 days after amputation. We also showed that intense cell proliferation occurs and is strictly required for regeneration to normally proceed.
In addition to its posterior body region, Platynereis worms can also successfully regenerate various types of body outgrowths, in particular their swimming/crawling appendages called parapodia. Platynereis is, in contrast, not able to regenerate its head after an amputation. While the worms can survive for many days after head amputation and a regeneration blastema is formed, the anterior blastema never produces any differentiated head structures (abortive regeneration).
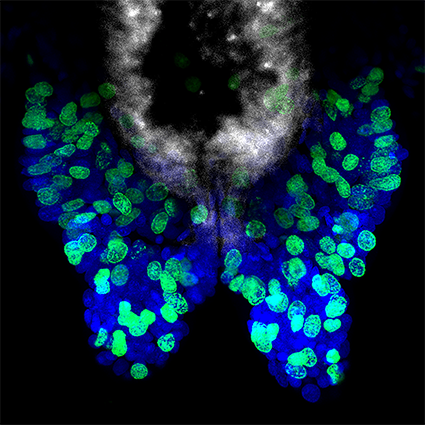
Figure 2: Posterior regeneration in Platynereis dumerilii. (Upper pannel) Bright-field microscopy images of the five well-defined stages of posterior regeneration. Brackets highlight the regenerated region whose size progressively increases during the different stages. (Lower panel) Expression at the different stages of regeneration of the Platynereis PCNA gene, a cell proliferation maker, which is expressed throughout regeneration. Images are from Planques et al., 2019.
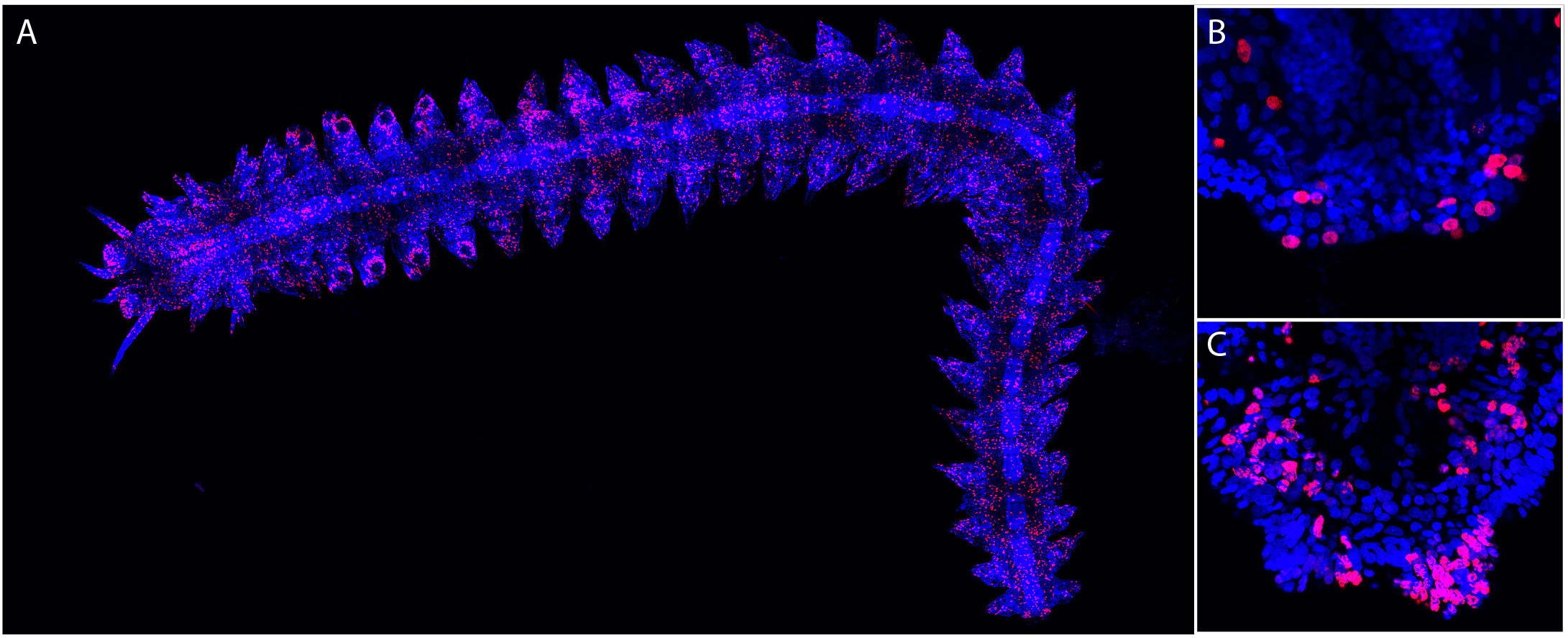
Figure 3: EdU labeling points out proliferating cells in Platynereis. EdU labeling (red) and Hoechst nuclear staining (blue) are shown. (A) Whole worm EdU labeling with 5h EdU labeling 24h after amputation (anterior is on the left). Many EdU+ cells are found throughout the body. (B) Higher-magnification view of the posterior part of the worm shown in (A), highlighting the presence of EdU+ cells in the regenerated region. (C) Posterior part of a worm after a 48h chase in normal sea water. Many EdU+ cells are found in the regenerated region. Images are from Planques et al., 2019.
Using a variety of approaches, we aim to better understand the amazing regenerative abilities of Platynereis and identify possible conserved mechanisms and genetic programs that underlie regeneration in animals. We also study the evolution of stem cells in animals. More specifically, our research projects are organized around five major axes:
(i) Origin(s) and fate(s) of the cells of the regeneration blastema in Platynereis. We obtained evidence that blastemal cells mainly derive from dedifferentiation of cells of the tissues abutting the amputation plane, which start, shortly after amputation, to express proliferation and pluripotent stem cell markers, suggesting that amputation induces extensive cell reprogramming towards a progenitor/stem cell identity (Planques et al., 2010; Bideau et al., in preparation). We are developing cell lineage and single-cell RNA-seq approaches to further characterize the dedifferentiation process and assess the identity and fate of the blastema cells.
(ii) Pathways involved in the initiation and control of posterior regeneration in Platynereis. We test the hypothesis that apoptosis and the production of reactive oxygen species (ROS) may be important to trigger regeneration, notably by stimulating cell proliferation required for regeneration, based on the knowledge obtained in other regenerative animals (reviewed in Vullien et al., 2021). We also study the potential role(s) of signaling pathways during regeneration and already identified two pathways (Notch and Wnt/beta-catenin) that are required for proper regeneration.
(iii) Comparison of the different regenerative processes in Platynereis. We aim to compare successful (posterior body and parapodia) and abortive (head) regeneration to identify mechanisms that are required for a successful regeneration. With this aim, we have started to characterize parapodia and head regeneration at the molecular, cellular and transcriptomic levels and compare the data obtained for these two regenerative events with those already obtained for posterior regeneration.
(iv) Epigenetic regulations during Platynereis regeneration. We obtained transcriptomic data that indicate that a large number of genes are differentially expressed during regeneration. We tested the hypothesis that epigenetic regulations may be involved in these dramatic changes in gene expression that occur after amputation. We obtained evidence for the involvement of DNA methylation and some histone modifications during regeneration (Planques et al., 2021; Schenkelaars et al., in preparation). We use epigenomic approaches (e.g., ATAC-seq and Cut&Tag) to further characterize this involvement and identify the gene regulatory network that sustains regeneration in Platynereis.
(v) Evolution of stem cells in animals. We previously showed that the growth of Platynereis worms relies on the presence of posterior stem cells that expressed pluripotent/germ stem cell markers (Gazave et al., 2013). We aim to define whether stem cells with a similar molecular signature might be present in other animals that progressively elongate their body, as Platynereis does. With this aim, we have started to study body growth in a chordate, the amphioxus Branchiostoma lanceolatum, and also plan to characterize growth in crustaceans. We are also characterizing how stem cells evolved in animals by defining and comparing the molecular signatures of a large variety of stem cells from different animals using published transcriptomic data.
Members
 Loeiza BADUEL, Biology technician, GAZAVE LAB+33 (0)1 57 27 81 01, room 293B
Loeiza BADUEL, Biology technician, GAZAVE LAB+33 (0)1 57 27 81 01, room 293B Yves CLEMENT, Assistant Professor, GAZAVE LAB+33 (0)1 57 27 80 03, room 283B
Yves CLEMENT, Assistant Professor, GAZAVE LAB+33 (0)1 57 27 80 03, room 283B Pierre KERNER, Assistant Professor, GAZAVE LAB+33 (0)1 57 27 20 24, room 283B
Pierre KERNER, Assistant Professor, GAZAVE LAB+33 (0)1 57 27 20 24, room 283B Brice PICHARD, PhD student, GAZAVE LAB+33 (0)1 57 27 81 01, room 293B
Brice PICHARD, PhD student, GAZAVE LAB+33 (0)1 57 27 81 01, room 293B Rémi STREHL, PhD student, GAZAVE LABroom 293B
Rémi STREHL, PhD student, GAZAVE LABroom 293B Zoé VELASQUILLO RAMIREZ, PhD student, GAZAVE LAB+33 (0)1 57 27 80 03, room 293B
Zoé VELASQUILLO RAMIREZ, PhD student, GAZAVE LAB+33 (0)1 57 27 80 03, room 293B
To contact a member of the team by e-mail: name.surname@ijm.fr
- Velaquillo 2025
- Bideau 2025,
- Vullien, A., Amiel, A. R., Baduel, L., Diken, D., Renaud, C., Krasovec, G., Vervoort, M., Röttinger, E., & Gazave, E. (2025). The Rich Evolutionary History of the Reactive Oxygen Species Metabolic Arsenal Shapes Its Mechanistic Plasticity at the Onset of Metazoan Regeneration. Molecular Biology and Evolution, 42(1), msae254. https://doi.org/10.1093/molbev/msae254
- Bideau, L., Velasquillo-Ramirez, Z., Baduel, L., Basso, M., Gilardi-Hebenstreit, P., Ribes, V., Vervoort, M., & Gazave, E. (2024). Variations in cell plasticity and proliferation underlie distinct modes of regeneration along the antero-posterior axis in the annelid Platynereis. Development, 151(20), dev202452. https://doi.org/10.1242/dev.202452
- Horkan, H. R., Popgeorgiev, N., Vervoort, M., Gazave, E., & Krasovec, G. (2024). Evolution of apoptotic signalling pathways within lophotrochozoans. Genome Biology and Evolution, evae204. https://doi.org/10.1093/gbe/evae204
- Álvarez-Campos, P., Planques, A., Bideau, L., Vervoort, M., & Gazave, E. (2023). On the hormonal control of posterior regeneration in the annelid Platynereis dumerilii. Journal of Experimental Zoology. Part B, Molecular and Developmental Evolution, 340(4), 298–315. https://doi.org/10.1002/jez.b.23182
- Bideau L, Kerner P, Hui J, Vervoort M, Gazave E. Animal regeneration in the era of transcriptomics. Cell Mol Life Sci. 2021 78(8):3941-3956. https://doi.org/10.1007/s00018-021-03760-7.
- Planques, A., Kerner, P., Ferry, L., Grunau, C., Gazave, E., & Vervoort, M. (2021). DNA methylation during development and regeneration of the annelid Platynereis dumerilii. BMC Biology, 19(1), 148. https://doi.org/10.1186/s12915-021-01074-5.
- Planques, A., Malem, J., Parapar, J., Vervoort, M., Gazave, E. (2019) Morphological, cellular and molecular characterization of posterior regeneration in the marine annelid Platynereis dumerilii. Dev Biol. 445(2):189-210. https://doi.org/10.1101/352211
- Gazave, E., Lemaître, Q. I. B., & Balavoine, G. (2017). The Notch pathway in the annelid Platynereis: insights into chaetogenesis and neurogenesis processes. Open Biology, 7(2), 160242. https://doi.org/10.1098/rsob.160242
Publications
Preprint
- Elena Simionato. Evolution et diversification des protéines à basic Helix-Loop-Helix chez les métazoaires : apports pour la compréhension de l’évolution du système nerveux et de la neurogenèse. Directeur de thèse : Michel Vervoort. Ecole doctorale GGC (Université Paris 11). Thèse soutenue le 24 novembre 2008. Mention Très Honorable.
- Pierre Kerner. Etude de l’évolution du système nerveux chez les animaux : neurogenèse comparative et phylogénomique. Directeur de thèse : Michel Vervoort. Ecole doctorale GGC (Université Paris 11). Thèse soutenue le 13 février 2009. Mention Très Honorable.
- Adrien Demilly. Génomique fonctionnelle et neurogenèse chez l’annélide Platynereis dumerilii. Directeur de thèse : Michel Vervoort. Ecole doctorale GC2iD (Université Paris 5/Université Paris 7). Thèse soutenue le 21 décembre 2012, Mention très Honorable avec félicitations du jury.
- Loïc Bideau. Régénération postérieure chez l’annélide Platynereis dumerilii: origine et devenir des cellules du blastème de régénération. Directrice de thèse : Eve Gazave. Ecole doctorale BioSPC (Université Paris Cité). Thèse soutenue le 6 Décembre 2023

- Aurore Vullien. Rôle des dérivés réactifs de l’oxygène (DRO) dans la régénération de structures complexes chez les annélides et les cnidaires. Directeurs de thèse : Eve Gazave et Eric Röttinger. Thèse en cotutelle entre Université Paris Cité (Ecole doctorale BioSPC) et Université de Nice (Ecole Doctorale des Sciences de la Vie et de la Santé). Thèse soutenue le 13 Septembre 2024

- Zoé Atzin Velasquillo Ramirez. Unraveling regeneration success in animals through a single-cell approach. Directrice de thèse : Eve Gazave. Ecole doctorale BioSPC (Université Paris Cité). Thèse soutenue le 8 Octobre 2025

- Brice Pichard. Régulations épigénétiques et épitranscriptomiques au cours du développement et de la régénération de l’annélide Platynereis dumerilii Directeurs de thèse : Eve Gazave et Pierre Kerner. Ecole doctorale BioSPC (Université Paris Cité). Thèse en cours (débutée le 1er octobre 2022).
- Rémi Strehl. Comprendre le succès de la régénération chez les animaux : apports de l’annélide Platynereis. Directrice de thèse : Eve Gazave. Ecole doctorale BioSPC (Université Paris Cité). Thèse en cours (débutée le 1er octobre 2025).
- Stéphanie Bertrand et Hector Escriva (Observatoire océanologique de Banyuls, France)
- Jerome Hui (The Chinese University of Hong Kong, Hong Kong)
- Lucie Laplane (IHPST et IGR, France)
- Eric Röttinger (IRCAN Nice, France)
- Arnau Sebé-Pedros (CRG Barcelona, Spain)
- Morgane Thomas-Chollier (IBENS, France)
- The Paris Region Fellowship – 2021
- Programme Emergence de la recherche de l’Idex Université de Paris – 2020
- CNRS INSB – Diversity of biological mechanisms – 2020
- CNRS IEA (France / Spain Collaboration) – 2020
- ANR PRC – 2020
- Fondation ARC pour la recherche sur le Cancer – 2019
- Labex Who AmI ? – 2019
- Ligue Nationale Contre le Cancer – 2019
- Programme PROCORE (France/Hong Kong collaboration) – 2019
- McDonnell foundation – 2019
- EMBRC France – 2018
- CNRS MITI – 2018
- ANR PRCI (ANR/FWF) – 2017
- Labex Who AmI ? – 2016
- PICS CNRS – 2015
- Labex Who AmI ? – 2014
- Labex Who AmI ? – 2013
- ANR “Programme blanc” – 2012
- Institut Universitaire de France (IUF) – 2010
03/03/25 Zoé Vélasquillo Ramirez reçeived the Young Researcher Award by the Fondation des Treilles


