Marianne Malartre
PhD – 2000-2004
- University of Portsmouth, UK. « Retinoic Acid Signalling and Primary Neuron Development » team (Colin Sharpe).
- Project: The role of nuclear receptor co-repressors in primary neurogenesis in Xenopus.
Postdoctoral research – 2004-2009
- Centro Andaluz de Biologia del Desarrollo (CABD), Seville, Spain.
- « Genetic and Cellular Mechanisms Regulating Cell Migration and Invasion » team (Lola Martin-Bermudo)
- Project: The role of the vav oncogene in the development of the Drosophila nervous system.
Associate professor, Paris-Saclay University – 2009-2024
- 2009-2013: INSERM UMR-S 757, « Calcium Signalling and Liver Regeneration » team (Laurent Combettes). Project: Calcium signalling during cell regeneration in Drosophila
- 2013-2024: I2BC (Institute of Integrative Cell Biology), « Cell Signalling and Morphogenesis » team (Anne-Marie Pret). Project: Role and regulation of the JAK/STAT signalling pathway during Drosophila oogenesis.
Professor, Paris Cité University – since 2024
- UMR7592, Jacques Monod Institute, « Comparative Developmental Neurobiology Laboratory » team (Nikos Konstantinides).
- Project: End of stemness during neurogenesis in the optic lobe of Drosophila
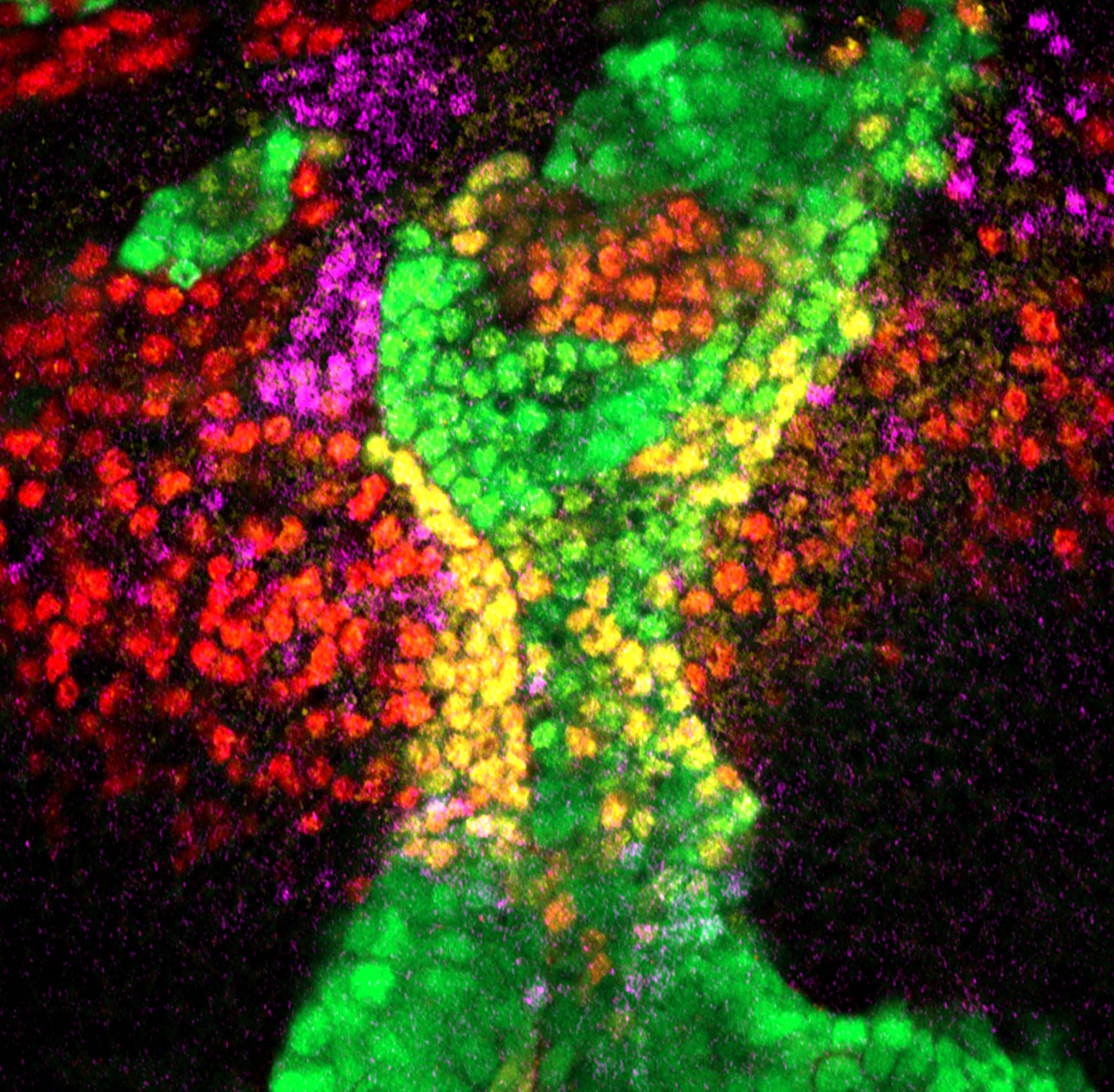
- Controlled arrest of neural stem cells is essential for brain development and for preventing excessive proliferation that could lead to tumours. In Drosophila, neural stem cells follow a precise temporal programme of transcription factor expression that generates neural diversity. Any disruption of this programme can extend the stemness state of these progenitors, delaying their elimination and potentially triggering over-proliferation. We are seeking to dissect how neuroblasts ensure a robust end to the stem state. In particular, we are exploiting a region of the optic lobe, the tOPC, a relatively simple system in which we have shown that neural stem cells can follow either a long or truncated temporal programme. We are currently seeking to identify the molecular mechanisms at work in varying the end of the stemness state of these neural progenitors, which will ultimately have consequences for neural diversity. Using the powerful genetic tools available in Drosophila, we aim to determine how these lineages terminate in different ways and what are the consequences upon defects in the termination of the stemness state.
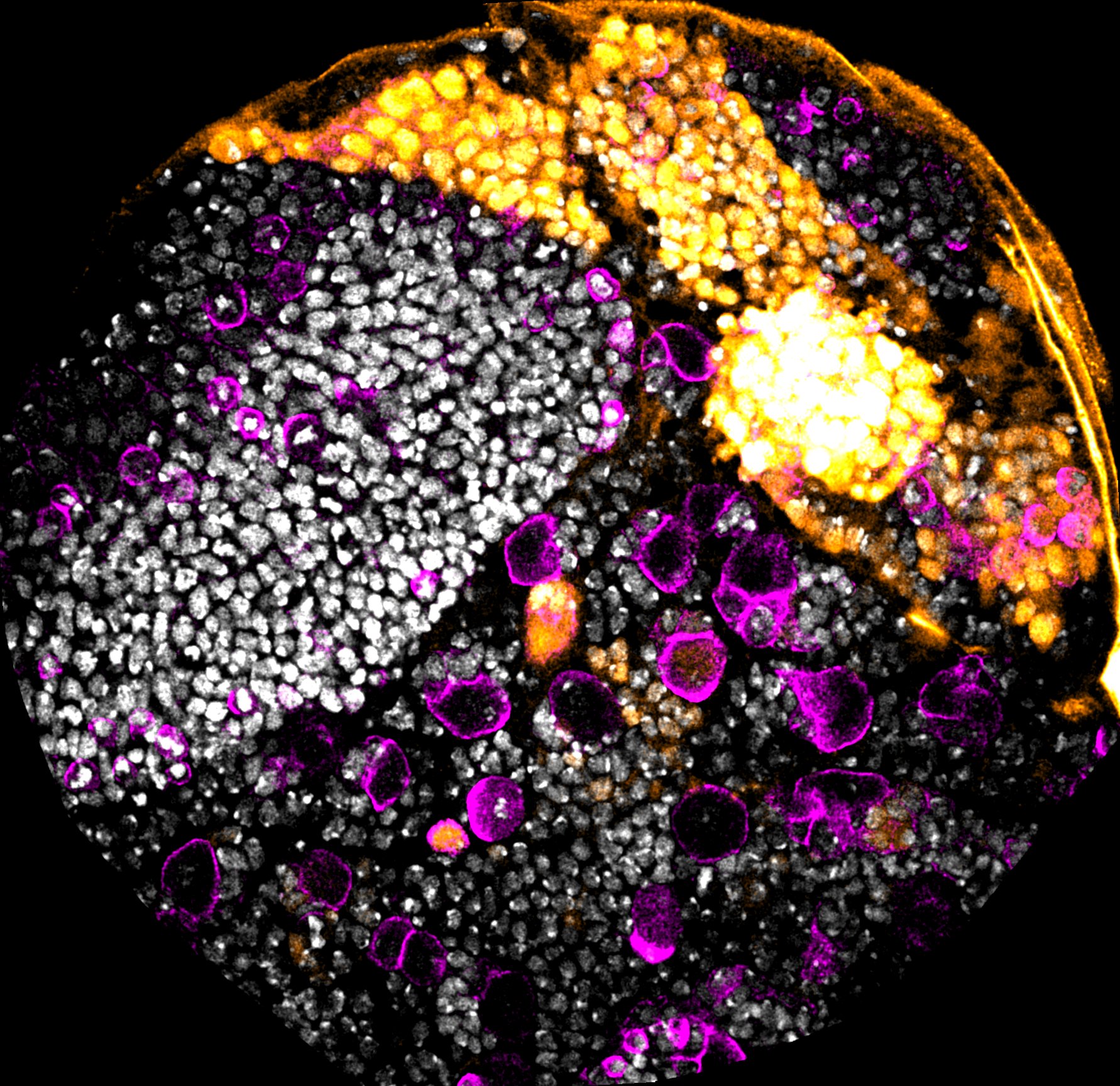
- In another part of the project, we are exploring the role of the environment on these same temporal series of transcription factors, which provide neural diversity. We would like to know whether environmental conditions such as changes in temperature or nutrition during development can have consequences on neural diversity, or whether mechanisms are set to protect the development of these cells in order to ensure the generation of all types of neurons in a robust manner.
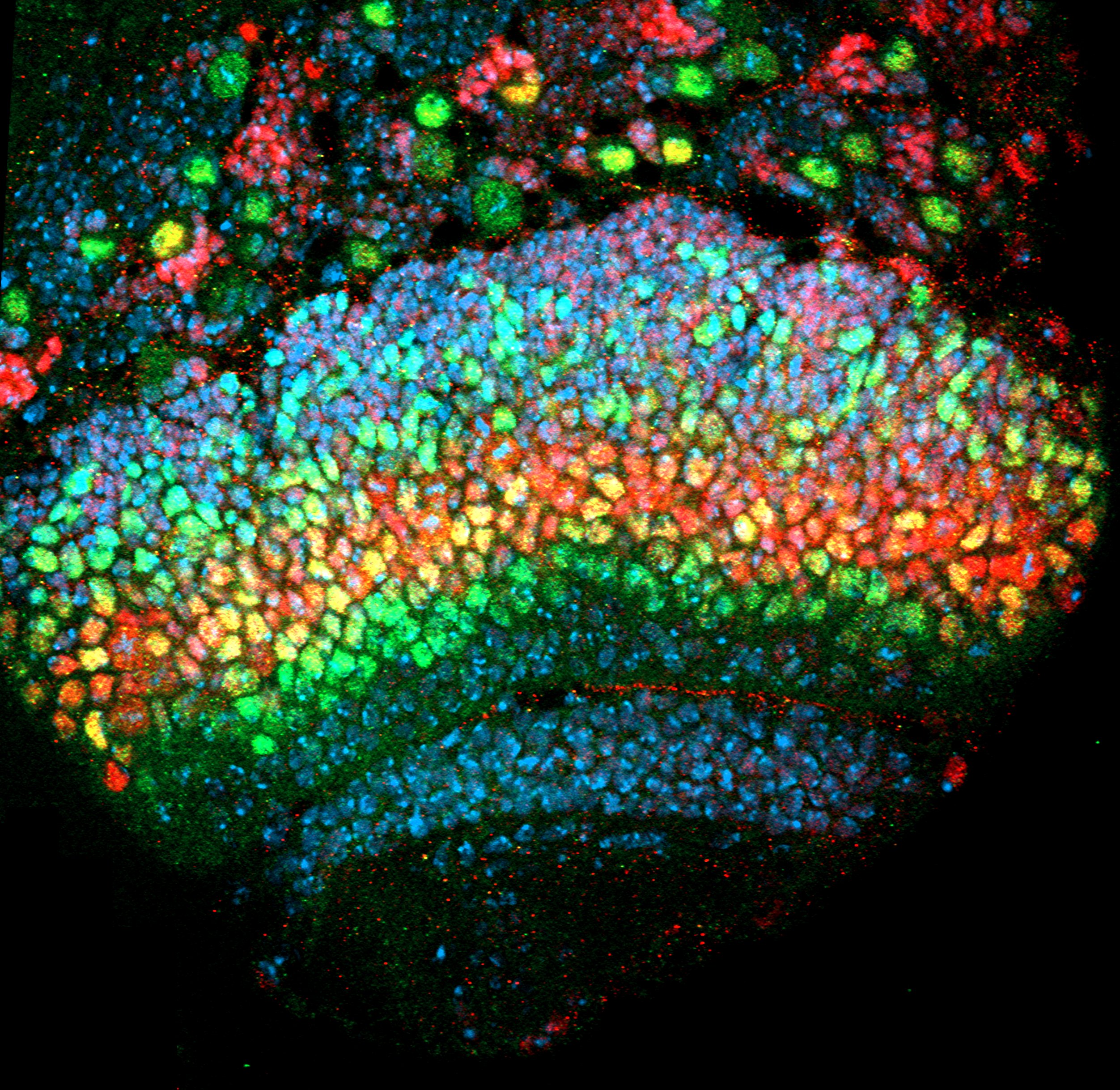
- 2018-2019 (2 ans) : La Ligue contre le Cancer (40k€). JAK-STAT signaling and epithelial integrity.
PhDs
- Marie-Charlotte Devagourou – 2025-2028 – End of stemness in the developing Drosophila brain.
- Kyriaki Niovi Rafailidou (co-supervised with Nikos Konstantinides) – 2025-2028 – Exploration of the full neurogenic potential of a brain structure
- Charlotte Mallart – 2018-2022 – Regulation and targets of the JAK-STAT pathway during Drosophila oogenesis.
- Laurine Miscopein-Saler (co-supervised with Anne-Marie Pret) – 2015-2016 – Role of the bric-à-brac locus during the formation of germ stem cell niches in the Drosophila ovary.
- Alba Torres (co-supervised with Francois Agnès and Anne-Marie Pret) – 2013-2016 – Link between JAK/STAT signalling, cellular remodelling and extrusion of a group of epithelial cells in the Drosophila ovary.
18 bachelor’s and master’s students – 2010-2025
- 8 second-year bachelor’s students; 2 Erasmus students (Portsmouth); 2 third-year bachelor’s students; 3 first-year master’s students; 3 second-year master’s students.
Paris-Saclay – 2009–2024
- Teaching at all levels from L1 to M2 in various modules on developmental biology, genetics, cell biology and scientific English.
Paris Cité – Since 2024
- L2: Genetics (tutorials, practicals); Evolutionary Biology (tutorials, practicals); Mechanisms of Development (lectures, tutorials)
- L3 Biodiversity and Ecosystems: Interface between Ecology, Evolution and Development (lectures)
- L3 Biosciences: Developmental Biology, Tools and Concepts (lectures, tutorials, practicals)
- L3 Magistère Européen de Génétique : Animal Developmental Biology (lectures, tutorials)
- M1 Neurosciences: Neurodevelopment and Evolution (Lectures, Tutorials)
- M1 Magistère Européen de Génétique : Developmental Genetics and Cell Biology (Lectures, Tutorials)
- M1 BMC: Molecular Biology of Development (Lectures, Tutorials)
- M2 GDC: Articles in English, Defence Panels
- M2 Magistère Européen de Génétique : Plasticity and Evolution of Development (Lectures, Tutorials)
https://orcid.org/0000-0003-2616-6976
Selected publications.
* corresponding author
2024 Mallart C., Netter S., Chalvet F., Montagne J., Claret S., Guichet A., Pret AM. and Malartre M.*. JAK-STAT-dependent contact between follicle cells and the oocyte controls Drosophila anterior-posterior polarity and germline development. Nat. Commun. 15, 1627.
Newsletters:
2022 Mallart C., Chalvet F., Sophie Netter, Torres A., Pret AM. and Malartre M.*. E-Cadherin acts as a positive regulator of the JAK-STAT signaling pathway during Drosophila oogenesis. Front. Cell Dev. Biol.. Aug 23;10:886312.
2020 Miscopein Saler L., Hauser V., Bartoletti M., Mallart C., Malartre M, Lebrun L., Pret AM Theodore L., Chalvet F. and Netter S. The Bric-à-Brac BTB/POZ transcription factors are necessary in niche cells for germline stem cells establishment and homeostasis through control of BMP/DPP signaling in the Drosophila melanogaster ovary. PLoS Genet. Nov 5;16(11):e1009128
2017 Yurani Torres A, Malartre M, Pret AM and Agnès F. JAK/STAT signaling is necessary for cell monosis prior to epithelial cell apoptotic extrusion. Cell Death Dis. May 25;8(5):e2814.
2016 Malartre M*. Regulatory mechanisms of EGFR signalling during Drosophila eye Development. Cell. Mol. Life Sci. 73:1825–1843.
Revue invitée.
2015 Martı́n-Bermudo MD, Bardet PL, Bellaïche Y, and Malartre M*. The vav oncogene antagonises EGFR signalling and regulates adherens junction dynamics during Drosophila eye development. Development. 142, 1-10.
- Sélectionné pour la couverture du journal.
- “Comment” dans Articles of Interest de J. Cell Science.
- Sélectionné comme Biomedical Picture of the Day (BPoD).

2013 Fernandez-Espartero C, Ramel D†, Farago M†, Malartre M†, Luque C†, Limanovich S, Katzav S, Emery G, and Martın-Bermudo MD. GTP exchange factor Vav regulates guided cell migration by coupling guidance receptor signalling to local Rac activation. J Cell Science. 126(10):2285-93
- Sélectionné pour la couverture du journal.
2010 Malartre M, Ayaz D, Amador FF, Martín-Bermudo MD. The guanine exchange factor vav controls axon growth and guidance during Drosophila development. J Neurosci. 30(6):2257-67.
- Featured article. “This week in the Journal: Role of vav in axon guidance”.

