Quantitative Biology of Bacterial Infection
Daria BONAZZI
Pathogenic bacteria can cross tissue barriers to invade and disseminate within the host. While numerous molecular mechanisms underlying these key infection steps have been identified, the physical forces involved—and their effects on infected cells, the extracellular matrix, and neighboring cells—remain poorly understood.
In our laboratory, we investigate these forces and examine how they influence both bacterial physiology and tissue mechanics. Our work primarily focuses on Neisseria meningitidis, an extracellular human-specific pathogenic bacterium responsible for severe diseases such as septic shock and meningitis. This organism is an ideal model for our studies, as meningococci rely on Type-IV pili to interact with their environment and with host tissues, generating strong pulling forces that can modulate host cell contractility.
To address these questions, we use a combination of live imaging, quantitative image analysis, microfabrication, microfluidics, and genetic approaches in in vitro cellular and tissue-barrier models of infection. Our research aims to elucidate how tissue mechanics and barrier function are altered when meningococci mechanically manipulate host tissues to promote bacterial dissemination and vascular damage.
This work serves a dual purpose: (i) to deepen our understanding of how mechanical forces contribute to infection, potentially informing new therapeutic strategies, and (ii) to advance fundamental knowledge of tissue-barrier mechanical homeostasis by leveraging extracellular pathogens as tools to uncover the complex feedback between cell architecture, mechanics, and function.
Keywords : mechanobiology, infection, Neisseria meningitidis, Type-IV pili, host/pathogen interaction, tissue barrier, cell mechanics, mechanotransduction, bacterial physiology, microscopy, microfabrication, microfluidics, vascular damage.
Introduction
Forces at the host/pathogen interface
Mammalian cells continuously sense and generate mechanical forces within their microenvironment to maintain tissue function, even during deformation and dynamic processes such as cell migration, division, and death. This mechanical resilience is especially important for epithelial and endothelial barriers, which must preserve integrity against pathogens.
Although mechanobiology has revealed key principles of tissue and microbial mechanics, it remains unclear how mechanical signals are integrated across scales to maintain tissue homeostasis during infection. Emerging studies show that pathogens can mechanically disrupt tissues, affecting infection outcomes, marking the rise of the “mechanobiology of infection.” This interdisciplinary area, positioned at the crossroads of microbiology, cell biology, and physics, now seeks to address key open questions: What forces operate at the interface between extracellular pathogens and the host? And how are these forces combined with bacteria–bacteria interactions within a colony?
The case of meningococcal infection, the role of tissue mechanics in barrier function
Neisseria meningitidis (Nm) is a powerful model for studying the mechanics of host–pathogen interactions because, during infection, its multicellular colonies encounter multiple tissue barriers, each with distinct mechanical properties. Normally a commensal of the nasopharyngeal epithelium, Nm can cause severe diseases such as septicemia and meningitis when it enters the bloodstream and crosses the blood–brain barrier. Throughout its infection cycle, Nm is exposed to various mechanical forces, including adhesion to epithelial or endothelial cells, forces generated by bacterial growth and aggregation, resistance to fluid flow, and forces involved in vascular leakage and dissemination.
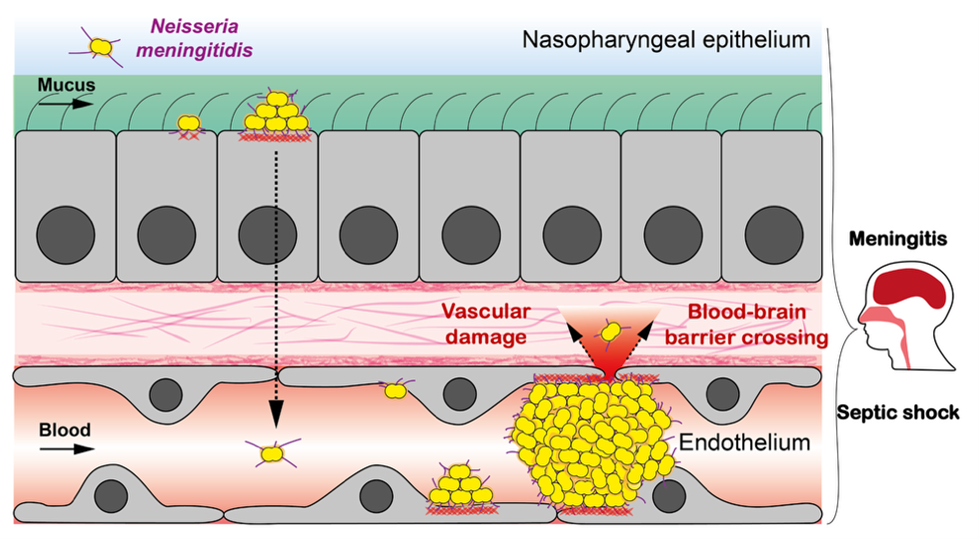
Schematics of Nm infection cycle. We aim at studying the potential interplay of mechanical forces in barrier breaching of epithelia and endothelia.
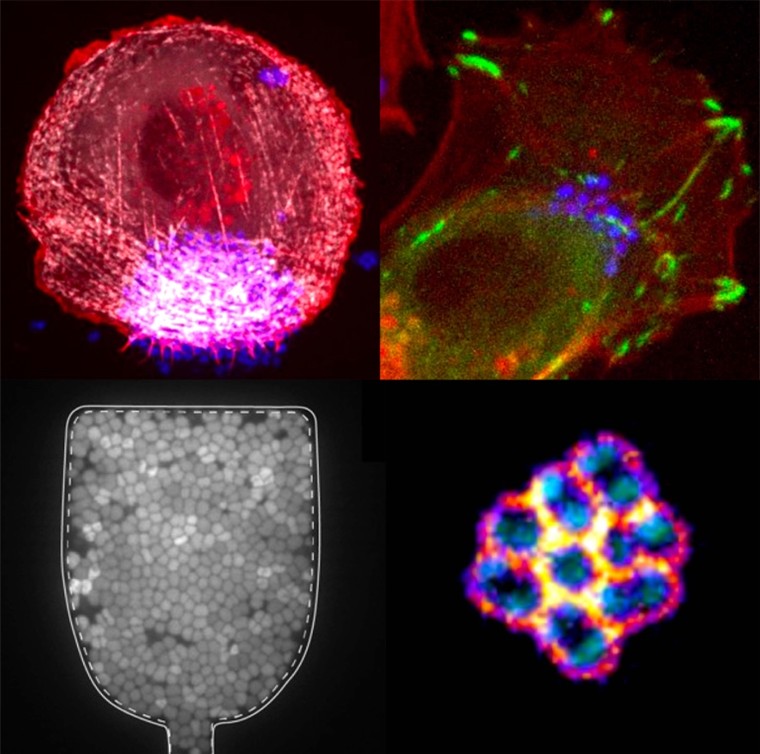
These processes depend on type IV pili (T4P), powerful retractile appendages. Prior work has shown that T4P dynamics generate intermittent interactions between bacteria, enabling the formation of cohesive, fluid-like aggregates that adapt to blood vessel geometry (Bonazzi et al, Cell, 2018). Nm also induces adhesion-driven spreading of the host membrane along T4P fibers (“1D-wetting”) (Charles-Orszag et al, Nat comm, 2018) and triggers the assembly of an actin-rich cortical plaque that stabilizes colonies under flow but weakens cell–cell junctions, promoting vascular damage.
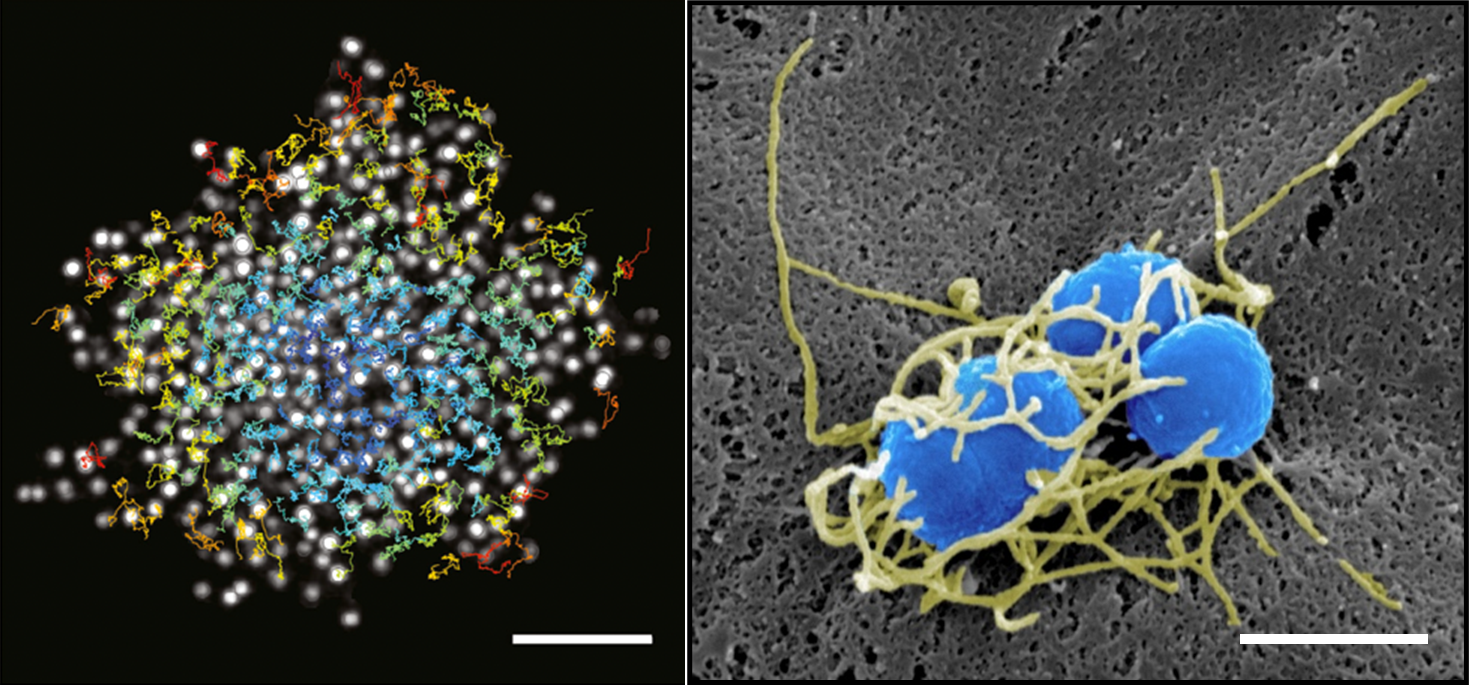
On the left, single confocal slice of a WT iRFP meningococcal aggregate in the middle plane and overlay of single bacterial tracks (color-coded by their mean instantaneous speeds). Scale bar, 10 μm. From Bonazzi et al, Cell, 2018. On the right, SEM image of a small bacterial colony, featuring a dense meshwork of Type-IV pili (false colored in yellow) that encloses the bacterial bodies (false colored in blue). Scale bar, 1 μm. From Charles-Orszag et al, Nat comm, 2018
Current projetcs
Control of Host Mechanics by a Bacterial Pathogen and Functional Impact
Recent work from the team revealed that Nm adhesion elicits strong, localized traction forces from endothelial cells, coordinated across the cell from the apical site of bacterial attachment to the basal substrate. This apico–basal mechanical communication involves a newly identified actin-rich structure, termed ancreopodia (Sankara et al, in preparation).
We are now investigating how extracellular adhesion of Nm alters host mechanics and function from the subcellular to the tissue scale. This includes studying forces directly exerted by bacteria – such as T4P-mediated adhesion and pulling – as well as host cell reprogramming and cytoskeletal changes that indirectly disrupt intercellular forces. The project aims to:
1) Identify key molecular players driving host cell remodeling during Nm infection and characterize their dynamics.
2) Measure mechanical changes in infected tissues using biophysical tools (e.g., traction force microscopy, FRET tension sensors, micropatterning, flow assays) and theoretical approaches.
3) Determine how infection affects cell behavior, tissue structure, and barrier integrity.
By considering both bacteria and host cells as active, force-generating systems, we aim to understand how bacterial adhesion alters cell mechanics, polarity, and motility, and how cytoskeletal remodeling disrupts cortical tension and intracellular organization, weakening tissue barriers.
Bacterial response to mechanical confinement
Within multicellular aggregates, bacterial proliferation occurs in a limited space, and in such environment, bacterial growth has been shown to generate internal mechanical stresses. This might occur in growing biofilms, but also during infection, when bacteria proliferate into intracellular compartments or tissular niches therefore imposing specific mechanical constraints. In the team, we investigate the forces generated upon bacterial growth under confinement, and their impact on bacterial physiology.
For this aim, we have successfully developed a PDMS-based microfluidic chip, enabling bacterial growth in a controlled mechanochemical environment (collaboration with M. Delarue, LAAS CNRS and G. Dumenil, Institut Pasteur). This innovative setup minimizes bacterial escape using submicron channels, facilitates efficient medium renewal, and allows for the direct measurement of growth-induced pressure. Using this system, we found that confinement of Escherichia coli generates very high compressive forces (hundreds of kPa), causing major changes in cell shape, growth, division, cytoplasmic properties, and gene expression. This response is conserved in a pathogenic E. coli strain causing urinary tract infections, suggesting that confinement-induced mechanical stress may contribute to disease progression, as supported by in vivo and ex vivo experiments. (collaboration with M. Ingersoll, Institut Cochin).
We are now studying the molecular mechanisms underlying transcriptional reprogramming in bacteria under mechanical constraints, focusing on its spatial-temporal dynamics at the single-cell level. For this, we are currently implementing large scale screenings techniques combined to a new generation of microfluidic chips that allow confinement of high bacterial numbers and cell retrieval afterwards. In parallel, we built a small library of fluorescent stress reporters to characterize spatial patterns of transcriptional activation inside the chamber and combine them to local pressure sensors.
Finally, we aim at extending these studies to other bacterial species (in particular to Nm, as we think bacteria might face mechanical constraints at late stages of vascular colonization, when they fill the entire vessel lumen) and assessing whether adaptation to confinement contributes to the emergence of antibiotic tolerance.
Cell morphogenesis and growth of a diplococcus
Bacteria robustly control their size and shape during their cell cycle. In the case of rod-shaped bacteria like Escherichia coli, high-throughput imaging coupled with image analysis tools yield an integrated view of the cell cycle and its regulation. However, for bacteria growing as diplococci, i.e. as two joined round cells, regulation of cell size, shape and division remains poorly understood.
In the team, we aim to provide a quantitative characterization of Nm cell cycle by building an efficient and robust image analysis pipeline for automated cell tracking and lineage analysis. We are investigating Nm dynamics of cellular components and the impact of chemical and mechanical stresses on these dynamics, both at single cell level and over several generations of bacteria. For this, two challenges at the interplay between imaging pathogens and data analysis need to be addressed: accurately measuring the subtle changes in shape of these small bacteria as they divide and proliferate, and integrating these single cell measurements into cell lineages that span a dozen division cycles.
This work will allow us to deploy analysis tools useful for the community of quantitative cell biology, to identify cell adaptation mechanisms that ensure cell survival during infection, and potentially to use cell shape control as a new target for disease treatment.
To contact a member of the team by e-mail: name.surname@ijm.fr
Laura Xénard (joint supervision with G. Dumenil and J.Y. Tinevez, Institut Pasteur)
Morgan Delarue (LAAS CNRS, Toulouse),
Guillaume Dumenil (institut Pasteur, Paris)
- ERC Starting