Hugo Wioland
Since 2020: CNRS researcher “Chargé de recherche” section 22
Institut Jacques Monod, CNRS, Université Paris Cité, France. Regulation of Cytoskeleton Dynamics lab (G. Romet-Lemonne & A. Jégou)
Role of tropomyosins in the differentiation of actin filament networks.
2015 – 2020: Post-doctoral fellow
Institut Jacques Monod, CNRS, Université Paris Cité, France. Regulation of Actin Assembly Dynamics lab (G. Romet-Lemonne & A. Jégou)
Regulation of actin filament disassembly by biochemical and mechanical factors.
2011-2015: PhD
DAMTP, University of Cambridge, United-Kingdom. Raymond E. Goldstein lab
Thèse “Self-Organization of Confined Active Matter”
I studied the collective motion of swimming bacteria inside novel microchambers.
My long term scientific project aims at understanding how diverse actin filament networks can emerge and coexist within a single cell. I am particularly interested in the many factors that make each actin filament unique, despite being assembled from the same pool of nearly identical actin monomers:
- Mechanical regulation: I have implemented a number of experimental setups to apply tension, torsion and curvature to single actin filaments, and shown for example that the actin binding protein ADF/cofilin exerts a torsion that boost filament fragmentation. I am now pursuing how individual and combined mechanical constraints can regulate protein binding to filaments.
- Actin post-translational modification: despite the fact that actin monomers can harbors a myriad of PTMs, their impact on actin dynamics has been barely studied. I have extensively studied actin Met44 and Met47 oxidation by the oxidoreductase MICAL1 and now aim at identifying and characterising other modifications.
- Tropomyosins: by covering >80% of filaments in cells, tropomyosins regulate the activity of many other actin binding proteins and thus defines the filaments’ identity. I am particularly interested in the factors that determine which tropomyosin isoform will cover actin.
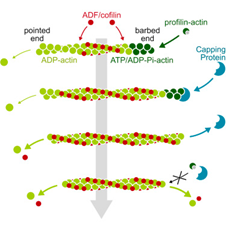
To better explore actin filament biochemical and biomechanical properties, we perform in vitro experiments with purified and fluorescently labelled proteins. Hundreds of single actin filaments are typically reconstituted inside a microfluidic chamber and exposed to solutions containing the proteins of interest. This allows us to precisely quantify actin filaments and their binding partners dynamics, and characterise the molecular interactions which shape filament networks inside cells.
Jan. 2026 – Déc 2029 | ANR PRC | 188 800 €
Partner in the MyoAssemble project (total 676 960 €), led by Anne Houdusse at the Institut Curie. Our goal is to characterize the atypical myosin18 which lacks an ATPase activity, with both structural and biophysical approaches.
Juin 2022 | Maupertius Short Mobility program | 1200 €
Supported by The Institut Français in Finland, I had the opportunity to visit the lab of Pekka Lappalainen (University of Helsinki) to establish a collaboration on the study of tropomyosins.
Jan. 2022 – Déc. 2025 | ANR JCJC | 320 000 €
Tropomyosins and differentiation of actin filament networks.
March 2015 – Feb. 2018 | Postdoctoral grant | Fondation ARC | Three year salary
- Anne Houdusse, Institut Curie | Funding: ANR PRC MyoAssemble
New collaboration on the atypical myosin 18.
- Pekka Lappalainen, University of Helsinki | Funding: Maupertius Short Mobility
Long-time collaboration on diverse actin binding proteins.
- Arnaud Echard et Stéphane Frémont, Institut Pasteur
Long-time collaboration on actin oxidation by the enzyme MICAL.
- Morgan Chabanon, CentraleSupélec
Recent collaboration to analyse our experiments with numerical simulations.
Engineer – Jean-Baptiste Berthelot | since 2025, ANR MyoAssemble – production and purification of cytoskeletal proteins
Postdoctoral fellow – Dina Al Abiad | 2023-2025, ANR TropActin – Biochemical regulation of Tropomyosin binding by Tropomodulin and actin PTMs.
PhD candidate – Camille Bagès | 2022-2026, ANR TropActin – Mechanical regulation of Tropomyosin and LIM-domain binding.
Internships – 5 master students and 4 bachelor students
Selected publication
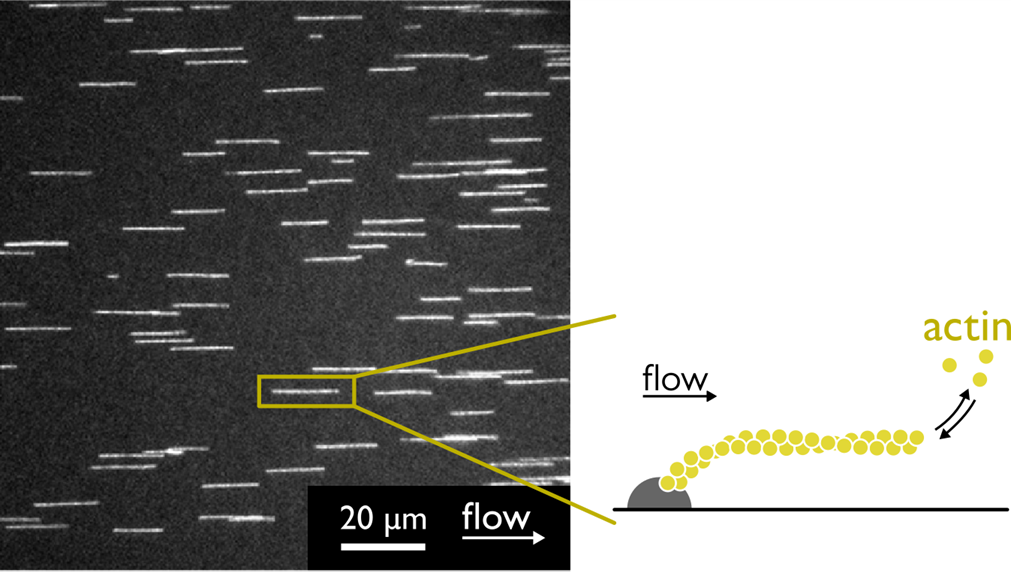
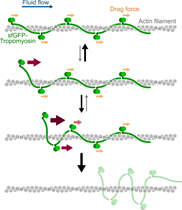
- Probing protein–protein interactions with drag flow: a case study of F-actin and tropomyosin, Bagès et al., European Journal of Physics E, 2025
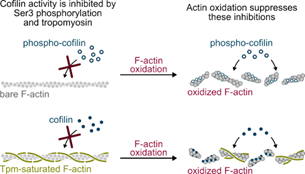
- Actin filament oxidation by MICAL1 suppresses protections from cofilin-induced disassembly, Wioland et al., EMBO Reports, 2021
- Torsional stress generated by ADF/cofilin on cross-linked actin filaments boosts their severing, Wioland et al., PNAS, 2019
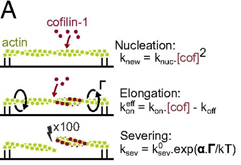
- ADF/cofilin accelerates actin dynamics by severing filaments and promoting their depolymerization at both ends, Wioland et al., Current Biology, 2017
- Ferromagnetic and antiferromagnetic order in bacterial vortex lattices, Wioland et al., Nature Physics, 2016