Véronique Brodu
Dynamics and function of the cytoskeleton in collective migration during morphogenesis of the tracheal system of the Drosophila embryo
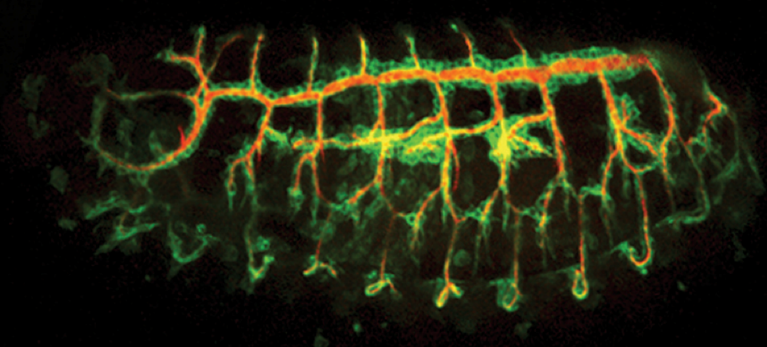
Organisation of the tracheal system (cells in green and lumen in red) in the Drosophila embryo
- 1996–2000: PhD in Invertebrate Physiology, University of Paris VI. Doctoral research conducted in Jean-Antoine LEPESANT’s team (Institut Jacques Monod, Paris, CNRS UMR 7592) under the supervision of Christophe ANTONIEWSKI.
- 2000–2003: Postdoctoral training, Laboratory of Dr. Alex GOULD, National Institute for Medical Research, MRC, London, UK.
- 2003–2006: Postdoctoral training, Laboratory of Prof. Jordi CASANOVA, Institute of Molecular Biology of Barcelona (IBMB), CSIC, Parc Científic de Barcelona, IRBB, Barcelona, Spain.
- 2006–present:Chargée de Recherche (First Class Research Scientist) at CNRS.
Member of the “Polarity and Morphogenesis” team led by Antoine GUICHET, Institut Jacques Monod, Paris, CNRS UMR 7592.
During development, tissues undergo extensive remodelling to shape organs, a process that often relies on collective cell migration. Interestingly, invasive cancers hijack similar mechanisms to spread into surrounding tissues. In organogenesis, epithelial remodelling involves changes in cell shape and position while maintaining stable contacts. Early in vitro models identified key principles of cell motility, now complemented by 3D studies showing how morphogenesis emerges from the interplay between actomyosin contractility and adhesion complexes that stabilize tissues.
Adherens junctions (AJs), built around the transmembrane protein E-Cadherin, play a central role. While ensuring cell cohesion, they must be dynamically reorganized. E-Cadherin mediates homophilic adhesion via its extracellular domain, while its intracellular domain connects to actomyosin and microtubule networks — both highly dynamic and essential for migration, adhesion, and mechanical stability.
To uncover fundamental processes of in vivo epithelial remodelling, we investigate the Drosophila tracheal system. This tubular network forms through stereotyped branching, driven by collective cell migration in response to FGF signalling. Remarkably, tracheal cells rearrange while maintaining permanent contacts, independently of Myosin II contractility. This makes the system a powerful model to identify novel mechanisms of junctional remodelling and intercalation.
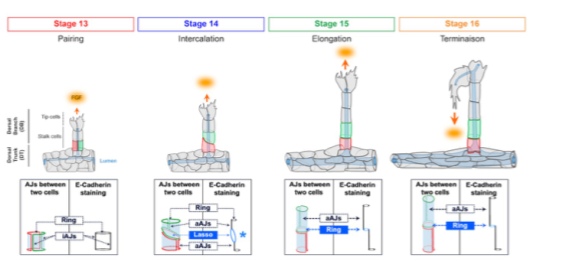
3D intercalation stages accompanying dorsal branch morphogenesis
Functional dynamics of microtubule network organization during collective migration of tracheal cells
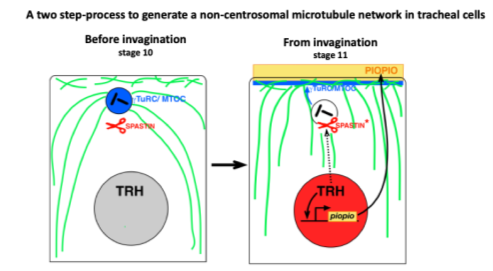
This project allowed us to characterise the organisation and nucleation of the microtubule network in tracheal cells. We demonstrated a change in the organisation of the microtubule nucleation centre. During tracheal morphogenesis, centrosomes are inactivated and microtubules are nucleated from the apical domain. This project characterised the role of microtubules during key steps associated with tissue remodelling: migration, intercalation, and the establishment of new cell–cell contacts.
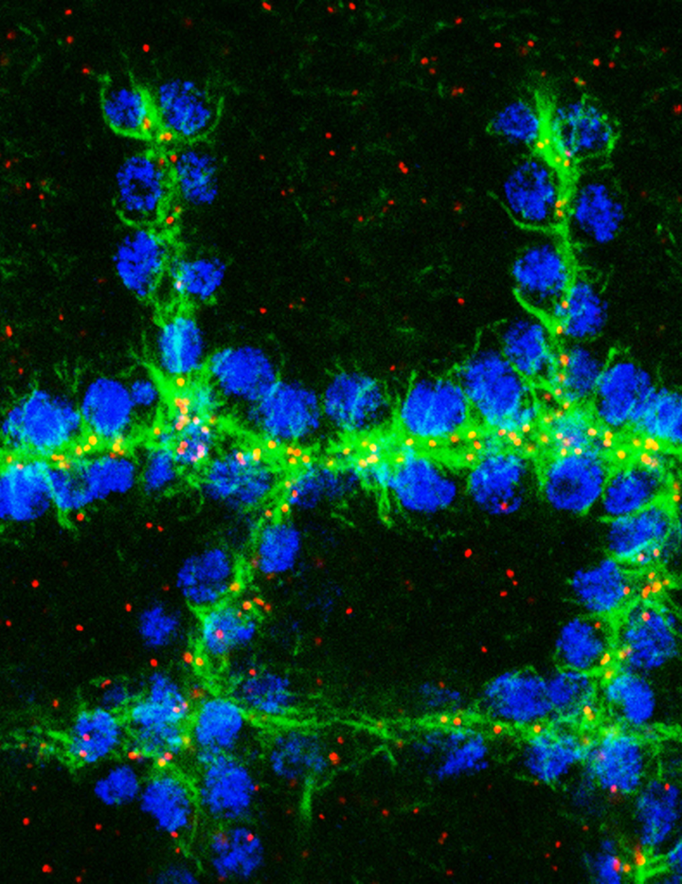
Organisation of the microtubule network (green) and position of centrioles (red) in tracheal cells (blue) at stage 14
Characterization of the actin cytoskeleton and its functional importance in cell intercalation during tracheal system morphogenesis
We aim to understand the molecular and cellular mechanisms by which the actin network generates the forces that enable cells to intercalate in vivo in 3D. To this end, we are developing innovative tools to monitor actin dynamics in vivo, determine its nature and origin, and assess its contribution to cell intercalation.
We characterized a progressive enrichment and stabilization of actin at adherens junctions (AJs) during intercalation. The cytoskeletal adaptor protein Girdin is a stable component of AJs, where it contributes to actin enrichment to ensure proper cell intercalation. Actin enrichment initiates at tricellular adherens junctions, where it exhibits a “pulsatile” behaviour, oscillating between phases of growth and decay.
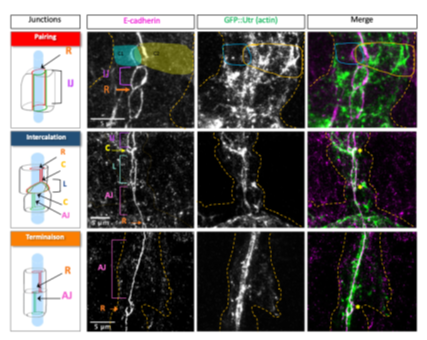
Distribution of the actin network (visualised by Utrophin::GFP) relative to AJs (marked by E-cadherin) in tracheal cells during dorsal branch morphogenesis.
- 2009–2011 – ARC Fixed Grant (with A. GUICHET), €50k / 2 years
- 2010–2015 – ARC Free Grant (with A. GUICHET), €450k / 3 years + 2-year extension
- 2023–2025 – Emergence en recherche grant, Université Paris Cité (with A. GUICHET & R. VIJENDRAVARMA), €34k / 2 years
- Cytoskeletal Dynamics Regulation group – Antoine JÉGOU & Guillaume ROMET-LEMONNE (Institut Jacques Monod, Paris)
- Cellular and Tissue Morphogenesis group – Manos MAVRAKIS & Loïc LE GOFF (Institut Fresnel, Marseille)
- Epithelial Morphogenesis group – Stefan LUSCHNIG (Institute of Cell Biology and Physiology, Münster, Germany)
- Mechanisms of Morphogenesis and Organogenesis group – Marta Llimargas (Institute of Molecular Biology of Barcelona, Spain)
PhD Supervision:
- 2009–2014: Co-supervision (with A. GUICHET) of the PhD thesis of Pierre-Marie LE DROGUEN, University Denis Diderot – Paris VII.
- 2022–present: Co-supervision (with A. GUICHET) of the PhD thesis of Sandra CARVALHO, University Paris Cité.
Master’s Thesis (M2) Supervision:
- 2009: Supervision of the M2 internship of Pierre-Marie LE DROGUEN, European Master’s in Genetics, University Denis Diderot – Paris VII.
- 2017–2018: Supervision of the M2 internship of Sarah TAHERALY, Master in Biology, Health and Ecology, specializationSignaling and Integrated Systems in Biology, EPHE.
- 2022: Supervision of the M2 internship of Sandra CARVALHO, Master in Biology and Cell Biology, University Paris Cité.
- 2023: Supervision of the M2 internship of Joanna AOUAD, Master in Life Sciences, specializationBiotechnology and Cell Engineering, University of Lorraine.
Master’s Thesis (M1) Supervision:
- 2007: Supervision of the M1 internship of Sophie MURAT, Master in Integrative Biology and Physiology, University Pierre and Marie Curie – Paris VI.
- 2012: Supervision of the M1 internship of Antonin OUDAR, Master inScience and Health, specialization Biomolecules and Experimental Therapies, University Paris XIII.
- 2016: Supervision of the M1 internship of Flora CROZET, Master in Molecular and Cellular Biology, University Pierre and Marie Curie – Paris VI.
- 2017: Supervision of the M1 internship of Fanny MARTINI, Master in Molecular and Cellular Biology, University Pierre and Marie Curie – Paris VI.
- 2017: Supervision of the M1 internship of Sarah ZEROUALI-BLONDEAU, Master in Biology and Health, specializationGenomics, Molecular and Cellular Biology, University Paris XI.
- 2019: Supervision of the free internship of Fanny WODRASCKA, M1 student, Master in Molecular and Cellular Biology, University of Paris.
- 2019: Supervision of the M1 internship of Mathilde CHOULY, Master in Molecular and Cellular Biology, University Pierre and Marie Curie – Paris VI.
- 2019–2020: Supervision of the M1 internship of Axel GOSSEYE, Master in Biology, Health and Ecology, specializationSignaling and Integrated Systems in Biology, EPHE.
- 2021: Supervision of the M1 internship of Benjamin BOUZERAND, Master in Molecular and Cellular Biology, University Paris Sorbonne.
- 2023: Supervision of the M1 internship of Mathilde LE GARFF, Master in Molecular and Cellular Biology, University of Rennes 1.
Other Supervision:
- 2015: Supervision of the L2 internship of Camille GUILLARD-SIRIEIX, Bachelor in Life Sciences, University Denis Diderot – Paris VII.
- 2017: Supervision of the 2nd-year BTS Biotechnology internship of Fanny WODRASCKA.
- 2010–present – Lecturer, M2 course Cellular Aspects of Development, Université Paris Cité
- 2020–present – Lecturer, M1 course Developmental Biology, ENS–Université PSL
Carvalho S., Laprise P., Guichet A., Brodu V*. (2025) Girdin controls the pace of 3D tracheal cell intercalation by coupling adherens junctions to the actin cytoskeleton in Drosophila, Development, in revision
Le Droguen P. M., Claret S., Guichet A*. and Brodu V*. (2015). Microtubule-dependent apical restriction of recycling endosomes sustains adherens junctions during morphogenesis of the Drosophila tracheal system. Development. 2015 142(2):363-74. * co-auteur correspondant
Brodu V*., Baffet A. D., Le Droguen P. M., Casanova J*. and Guichet A. (2010), A developmentally regulated two−step process generates a non−centrosomal microtubule network in Drosophila tracheal cells. Developmental Cell 18(5):790−801. * co-auteur correspondant
Brodu V. and Casanova J. (2006). The RhoGAP crossveinless-c links trachealess and EGFR signalling to cell shape remodelling in Drosophila tracheal invagination. Genes and Development, 20, 1817−1828
Brodu V., Elstob P.E. and Gould A.P. (2004) EGF Receptor Signaling is required for pulses of cell delamination from the Drosophila ectoderm. Developmental Cell, 7, 885-895
Brodu V., Elstob P.E. and Gould A.P. (2002) abdominal A specifies one cell identity in Drosophila by regulating one principal target gene. Development, 129, 2957-2963.
Elstob P.E., Brodu V. and Gould A.P. (2001) spalt-dependent switching between two cell fates that are induced by the Drosophila EGF receptor. Development, 128, 723-732.
Gould A.P., Elstob P.E. and Brodu V. (2001) Insect oenocytes: a system for studying cell-fate specification by Hox genes. Journal of Anatomy, 199, 25-33.
Brodu V., Mugat M., Fichelson P., Lepesant J.A. and Antoniewski C. (2001) A UAS site substitution approach to the in vivo dissection of promoters : interplay betwwen the GATAb activator and the AEF-1 repressor at a Drosophila Ecdysone Reponse Unit. Development, 128, 2593-2602.
September 2008–present: Initiator and coordinator of the “Cytoskeleton Club”. This monthly meeting brings together different laboratories around a common research theme, the cytoskeleton. Over 17 seasons, it has gathered 44 teams — about 320 people — from 18 research institutes.
November 2020, November 2021, June 2023, November 2024, and November 2025: Co-organiser of a full-day event, “Paris Cytoskeleton Day”, at the Institut Jacques Monod, bringing together national and international speakers on the topic of the cytoskeleton.

