Recherche
La compréhension des mécanismes qui orchestrent la formation de tissus et d’organes et qui contrôlent le maintien de leur architecture est une question fondamentale en biologie. La formation des tissus et leur homéostasie sont coordonnées par des processus cellulaires incluant la polarité cellulaire, l’adhérence et la motilité. La compréhension de ces processus est aussi essentielle pour mieux comprendre le développement de pathologies comme le cancer.
Notre équipe de recherche vise à élucider les mécanismes qui contrôlent la polarité cellulaire et la morphogenèse tissulaire en relation avec le cytosquelette et en particulier avec le réseau de microtubules. Pour cela nous utilisons le développement de la drosophile comme modèle d’étude et nous concentrons notre recherche sur deux axes.
- Au niveau cellulaire, en étudiant le rôle du cytosquelette dans la mise en place de la polarité de l’ovocyte au cours de l’ovogenèse. Nous cherchons à déterminer les mécanismes impliqués dans le transport asymétrique de protéines et d’ARNs et ceux impliqués dans le positionnement asymétrique du noyau.
- Au niveau tissulaire, en étudiant l’implication du cytosquelette dans la morphogenèse tissulaire. Nous cherchons à identifier les mécanismes contrôlant la migration collective de cellules, assurant la formation du système respiratoire au cours de l’embryogénèse.
Les approches expérimentales utilisées au laboratoire combinent la génétique, la biophysique et des techniques de biologie cellulaire. De plus, la microscopie photonique de pointe sur tissus vivants ainsi que la microscopie électronique constituent le cœur de nos expériences quotidiennes.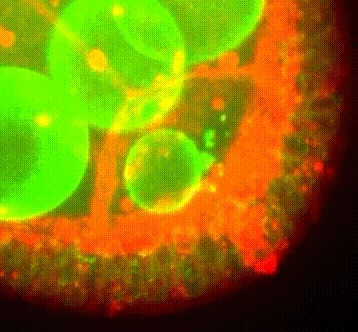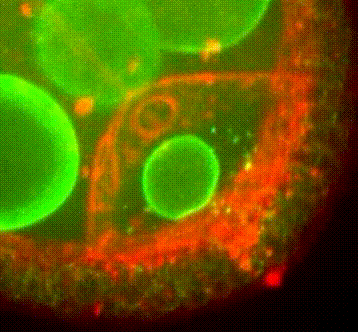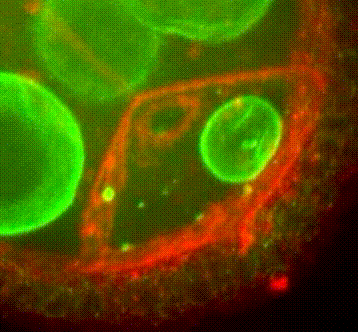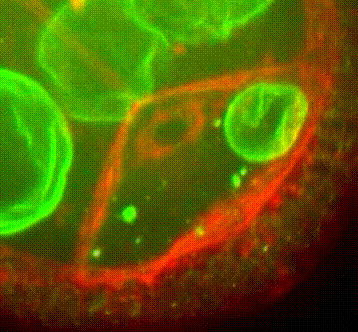
Migration du noyau dans l’ovocyte de drosophile
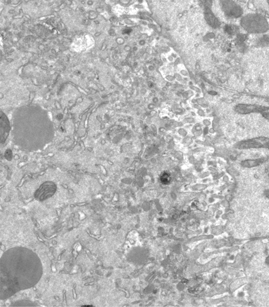
Organisation des membranes dans l’ovocyte de drosophile
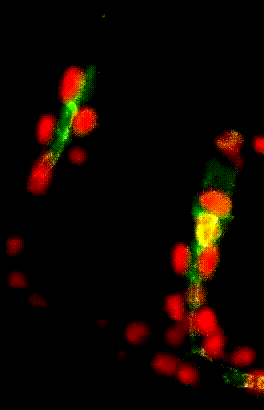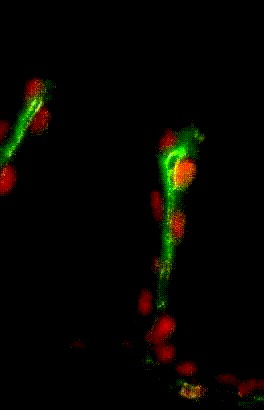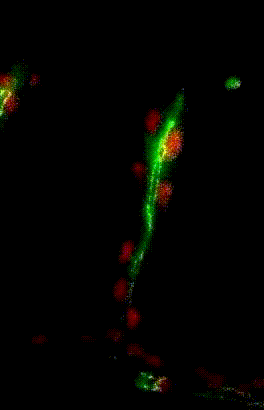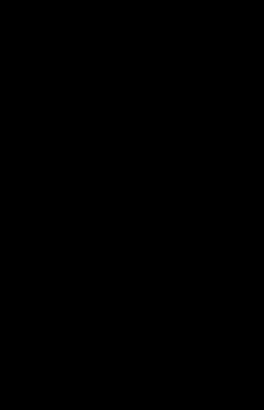
Migration collectives des cellules trachéales dans l’embryon de drosophile
Membres
Responsable
Antoine GUICHET,
Researcher,
GUICHET LAB+33 (0)1 57 27 80 76, bureau 422B
Membres
Frédéric BERNARD,
Assistant Professor,
GUICHET LAB+33 (0)1 57 27 80 77, bureau 422B
Veronique BRODU,
Researcher,
GUICHET LAB+33 (0)1 57 27 80 78, bureau 422B
Sylvain BRUN,
Assistant Professor,
GUICHET LAB+33 (0)1 57 27 80 87, bureau 422B
Sandra CARVALHO,
PhD student,
GUICHET LAB+33 (0)1 57 27 80 76, bureau 422B
Sandra CLARET,
Assistant Professor,
GUICHET LAB+33 (0)1 57 27 80 77, bureau 422B
Zoe KACHANER,
Intern,
GUICHET LAB Jean-Antoine LEPESANT,
Emeritus researcher,
GUICHET LAB+33 (0)1 57 27 80 78, bureau 422B
Maxime PONTHIER,
Intern,
GUICHET LAB Fanny ROLAND-GOSSELIN,
PhD student,
GUICHET LAB+33 (0)1 57 27 80 77, bureau 422B
Wiam SINDABAD,
Intern,
GUICHET LAB
Pour contacter un membre de l’équipe par mail : prenom.nom@ijm.fr
Sélection de publications
The Importance of the Position of the Nucleus in Drosophila Oocyte Development. Lepesant JA, Roland-Gosselin F, Guillemet C, Bernard F, Guichet A. Cells. (2024)
Kinesin-1 promotes centrosome clustering and nuclear migration in the Drosophila oocyte. Development. Loh, M., Bernard, F., Guichet, A. (2023).
Dynein-mediated transport and membrane trafficking control PAR3 polarised distribution. Jouette J, Guichet A, Claret S. eLIFE (2019)
Distinct molecular cues ensure a robust microtubule-dependent nuclear positioning in the Drosophila oocyte.Tissot N, Lepesant JA, Bernard F, Legent K, Bosveld F, Martin C, Faklaris O, Bellaïche Y, Coppey M, Guichet A. Nature Communication. (2017)
Microtubule-dependent apical restriction of recycling endosomes sustains adherens junctions during morphogenesis of the Drosophila tracheal system. Le Droguen PM, Claret S, Guichet A, Brodu V. Development. (2015)
PI(4,5)P2 produced by the PI4P5K Skittles controls the apical domain size by tethering PAR-3 in Drosophila epithelial cells. Claret S, Benoit B, Richard-Ferrec G, Guichet A, Current Biology. (2014)
A developmentally regulated two-step process generates a non-centrosomal microtubule network. Brodu V, Baffet A, Le Droguen PM, Casanova J, Guichet A. Developmental Cell . (2010)
PIP5K-dependent production of PIP2 sustains microtubule organization to establish polarized transport in the Drosophila oocyte. Gervais L, Claret S, Januschke J, Roth S, Guichet A. Development (2008).
The Centrosome Nucleus complex directs the formation of two orthogonal microtubule polarized transport in the Drosophila oocyte Januschke J, Gervais L, Gillet, L., Keryer G, Bornen M, Guichet A. Development. (2006).
Polar transport in the Drosophila oocyte requires Dynein and Kinesin I cooperation, Januschke J, Gervais L, Dass S, Kaltschmidt J, Lopez-Schier H, St. Johnston D, Brand A, Roth S and Guichet A. Current Biology (2002).
Toutes les publications depuis 2017
Publications
2913254
UAHRRGCT
1
apa
50
date
desc
8720
https://www.ijm.fr/wp-content/plugins/zotpress/
%7B%22status%22%3A%22success%22%2C%22updateneeded%22%3Afalse%2C%22instance%22%3Afalse%2C%22meta%22%3A%7B%22request_last%22%3A0%2C%22request_next%22%3A0%2C%22used_cache%22%3Atrue%7D%2C%22data%22%3A%5B%7B%22key%22%3A%22RMMEDK4Y%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Guyot%20et%20al.%22%2C%22parsedDate%22%3A%222025-06-19%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BGuyot%2C%20L.%2C%20Chahine%2C%20E.%2C%20De%20Filippo%2C%20E.%2C%20Lalanne%2C%20C.%2C%20Brun%2C%20S.%2C%20Hartmann%2C%20F.%20E.%2C%20%26amp%3B%20Giraud%2C%20T.%20%282025%29.%20Sheltered%20load%20in%20fungal%20mating-type%20chromosomes%20revealed%20by%20fitness%20experiments.%20%26lt%3Bi%26gt%3BJournal%20of%20Evolutionary%20Biology%26lt%3B%5C%2Fi%26gt%3B%2C%20voaf079.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1093%5C%2Fjeb%5C%2Fvoaf079%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1093%5C%2Fjeb%5C%2Fvoaf079%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Sheltered%20load%20in%20fungal%20mating-type%20chromosomes%20revealed%20by%20fitness%20experiments%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Lou%22%2C%22lastName%22%3A%22Guyot%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Elizabeth%22%2C%22lastName%22%3A%22Chahine%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Elsa%22%2C%22lastName%22%3A%22De%20Filippo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christophe%22%2C%22lastName%22%3A%22Lalanne%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sylvain%22%2C%22lastName%22%3A%22Brun%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fanny%20E%22%2C%22lastName%22%3A%22Hartmann%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Tatiana%22%2C%22lastName%22%3A%22Giraud%22%7D%5D%2C%22abstractNote%22%3A%22Sex%20chromosomes%20and%20mating-type%20chromosomes%20can%20carry%20large%20regions%20with%20suppressed%20recombination.%20As%20a%20result%20of%20a%20lower%20efficacy%20of%20selection%2C%20recessive%20deleterious%20mutations%20are%20expected%20to%20accumulate%20in%20these%20non-recombining%20regions.%20Multiple%20genomic%20analyses%20have%20indirectly%20inferred%20the%20presence%20of%20deleterious%20mutations%20in%20sex%20and%20mating-type%20chromosomes%2C%20but%20direct%20experimental%20evidence%20remains%20scarce.%20Here%2C%20we%20performed%20fitness%20assays%20in%20fungi%20with%20megabase-large%20and%20young%20non-recombining%20regions%20around%20the%20mating-type%20locus%2C%20using%20three%20Sordariales%20species%2C%20to%20test%20whether%20heterokaryons%20%28diploid-like%2C%20heterozygous%20at%20the%20mating-type%20locus%29%20exhibited%20a%20fitness%20advantage%20over%20homokaryons%20%28haploid-like%2C%20with%20a%20single%20mating-type%20allele%29%2C%20in%20terms%20of%20spore%20germination%20dynamics%20or%20mycelium%20growth%20speed%2C%20under%20different%20conditions%20of%20light%20and%20temperature.%20We%20found%20a%20faster%20growth%20of%20heterokaryons%20compared%20to%20one%20of%20the%20homokaryons%20for%20Podospora%20anserina%20at%2018%5Cu00b0C%20and%20for%20Schizothecium%20tetrasporum%20and%20Schizothecium%20tritetrasporum%20at%2022%5Cu00b0C%20under%20light.%20These%20findings%20suggest%20the%20presence%20of%20a%20sheltered%20load%2C%20i.e.%2C%20recessive%20deleterious%20mutations%20at%20the%20heterozygous%20state%20in%20or%20near%20non-recombining%20regions%2C%20associated%20to%20a%20specific%20mating-type%20allele.%20Genomic%20analyses%20indeed%20suggested%20that%20the%20non-recombining%20regions%20around%20the%20mating-type%20locus%20likely%20carries%20heterozygous%20deleterious%20mutations%2C%20while%20the%20rest%20of%20the%20genome%20was%20mostly%20homozygous.%20We%20also%20showed%20that%20the%20difference%20in%20growth%20rates%20did%20not%20result%20from%20different%20numbers%20or%20densities%20of%20nuclei%20between%20homokaryons%20and%20heterokaryons.%20Leveraging%20the%20experimental%20assets%20of%20fungi%2C%20allowing%20cultivating%20separately%20haploid-like%20and%20diploid-like%20life%20stages%2C%20our%20experiments%20provided%20one%20of%20the%20rare%20direct%20experimental%20evidence%20of%20sheltered%20load%20around%20mating-compatibility%20loci%2C%20which%20is%20crucial%20for%20our%20understanding%20of%20sex-related%20chromosome%20evolution.%22%2C%22date%22%3A%222025-06-19%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1093%5C%2Fjeb%5C%2Fvoaf079%22%2C%22ISSN%22%3A%221010-061X%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1093%5C%2Fjeb%5C%2Fvoaf079%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222025-06-30T09%3A44%3A55Z%22%7D%7D%2C%7B%22key%22%3A%22YNSD6AFM%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Mallart%20et%20al.%22%2C%22parsedDate%22%3A%222024-02-22%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BMallart%2C%20C.%2C%20Netter%2C%20S.%2C%20Chalvet%2C%20F.%2C%20Claret%2C%20S.%2C%20Guichet%2C%20A.%2C%20Montagne%2C%20J.%2C%20Pret%2C%20A.-M.%2C%20%26amp%3B%20Malartre%2C%20M.%20%282024%29.%20JAK-STAT-dependent%20contact%20between%20follicle%20cells%20and%20the%20oocyte%20controls%20Drosophila%20anterior-posterior%20polarity%20and%20germline%20development.%20%26lt%3Bi%26gt%3BNature%20Communications%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B15%26lt%3B%5C%2Fi%26gt%3B%281%29%2C%201627.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1038%5C%2Fs41467-024-45963-z%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1038%5C%2Fs41467-024-45963-z%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22JAK-STAT-dependent%20contact%20between%20follicle%20cells%20and%20the%20oocyte%20controls%20Drosophila%20anterior-posterior%20polarity%20and%20germline%20development%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Charlotte%22%2C%22lastName%22%3A%22Mallart%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sophie%22%2C%22lastName%22%3A%22Netter%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fabienne%22%2C%22lastName%22%3A%22Chalvet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sandra%22%2C%22lastName%22%3A%22Claret%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jacques%22%2C%22lastName%22%3A%22Montagne%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anne-Marie%22%2C%22lastName%22%3A%22Pret%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Marianne%22%2C%22lastName%22%3A%22Malartre%22%7D%5D%2C%22abstractNote%22%3A%22The%20number%20of%20embryonic%20primordial%20germ%20cells%20in%20Drosophila%20is%20determined%20by%20the%20quantity%20of%20germ%20plasm%2C%20whose%20assembly%20starts%20in%20the%20posterior%20region%20of%20the%20oocyte%20during%20oogenesis.%20Here%2C%20we%20report%20that%20extending%20JAK-STAT%20activity%20in%20the%20posterior%20somatic%20follicular%20epithelium%20leads%20to%20an%20excess%20of%20primordial%20germ%20cells%20in%20the%20future%20embryo.%20We%20show%20that%20JAK-STAT%20signaling%20is%20necessary%20for%20the%20differentiation%20of%20approximately%2020%20specialized%20follicle%20cells%20maintaining%20tight%20contact%20with%20the%20oocyte.%20These%20cells%20define%2C%20in%20the%20underlying%20posterior%20oocyte%20cortex%2C%20the%20anchoring%20of%20the%20germ%20cell%20determinant%20oskar%20mRNA.%20We%20reveal%20that%20the%20apical%20surface%20of%20these%20posterior%20anchoring%20cells%20extends%20long%20filopodia%20penetrating%20the%20oocyte.%20We%20identify%20two%20JAK-STAT%20targets%20in%20these%20cells%20that%20are%20each%20sufficient%20to%20extend%20the%20zone%20of%20contact%20with%20the%20oocyte%2C%20thereby%20leading%20to%20production%20of%20extra%20primordial%20germ%20cells.%20JAK-STAT%20signaling%20thus%20determines%20a%20fixed%20number%20of%20posterior%20anchoring%20cells%20required%20for%20anterior-posterior%20oocyte%20polarity%20and%20for%20the%20development%20of%20the%20future%20germline.%22%2C%22date%22%3A%222024-02-22%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1038%5C%2Fs41467-024-45963-z%22%2C%22ISSN%22%3A%222041-1723%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.nature.com%5C%2Farticles%5C%2Fs41467-024-45963-z%22%2C%22collections%22%3A%5B%22EWC7IWVP%22%2C%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-27T09%3A34%3A56Z%22%7D%7D%2C%7B%22key%22%3A%22UYGGYY3H%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Lepesant%20et%20al.%22%2C%22parsedDate%22%3A%222024-01%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BLepesant%2C%20J.-A.%2C%20Roland-Gosselin%2C%20F.%2C%20Guillemet%2C%20C.%2C%20Bernard%2C%20F.%2C%20%26amp%3B%20Guichet%2C%20A.%20%282024%29.%20The%20Importance%20of%20the%20Position%20of%20the%20Nucleus%20in%20Drosophila%20Oocyte%20Development.%20%26lt%3Bi%26gt%3BCells%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B13%26lt%3B%5C%2Fi%26gt%3B%282%29%2C%20201.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fcells13020201%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fcells13020201%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22The%20Importance%20of%20the%20Position%20of%20the%20Nucleus%20in%20Drosophila%20Oocyte%20Development%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jean-Antoine%22%2C%22lastName%22%3A%22Lepesant%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fanny%22%2C%22lastName%22%3A%22Roland-Gosselin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Cl%5Cu00e9mentine%22%2C%22lastName%22%3A%22Guillemet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fred%22%2C%22lastName%22%3A%22Bernard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%5D%2C%22abstractNote%22%3A%22Oogenesis%20is%20a%20developmental%20process%20leading%20to%20the%20formation%20of%20an%20oocyte%2C%20a%20haploid%20gamete%2C%20which%20upon%20fertilisation%20and%20sperm%20entry%20allows%20the%20male%20and%20the%20female%20pronuclei%20to%20fuse%20and%20give%20rise%20to%20a%20zygote.%20In%20addition%20to%20forming%20a%20haploid%20gamete%2C%20oogenesis%20builds%20up%20a%20store%20of%20proteins%2C%20mRNAs%2C%20and%20organelles%20in%20the%20oocyte%20needed%20for%20the%20development%20of%20the%20future%20embryo.%20In%20several%20species%2C%20such%20as%20Drosophila%2C%20the%20polarity%20axes%20determinants%20of%20the%20future%20embryo%20must%20be%20asymmetrically%20distributed%20prior%20to%20fertilisation.%20In%20the%20Drosophila%20oocyte%2C%20the%20correct%20positioning%20of%20the%20nucleus%20is%20essential%20for%20establishing%20the%20dorsoventral%20polarity%20axis%20of%20the%20future%20embryo%20and%20allowing%20the%20meiotic%20spindles%20to%20be%20positioned%20in%20close%20vicinity%20to%20the%20unique%20sperm%20entry%20point%20into%20the%20oocyte.%22%2C%22date%22%3A%222024%5C%2F1%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.3390%5C%2Fcells13020201%22%2C%22ISSN%22%3A%222073-4409%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.mdpi.com%5C%2F2073-4409%5C%2F13%5C%2F2%5C%2F201%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-01-29T12%3A39%3A10Z%22%7D%7D%2C%7B%22key%22%3A%22R5K2RH3V%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Zhu%20et%20al.%22%2C%22parsedDate%22%3A%222023-09-12%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BZhu%2C%20Z.%2C%20Becam%2C%20I.%2C%20Tovey%2C%20C.%20A.%2C%20Elfarkouchi%2C%20A.%2C%20Yen%2C%20E.%20C.%2C%20Bernard%2C%20F.%2C%20Guichet%2C%20A.%2C%20%26amp%3B%20Conduit%2C%20P.%20T.%20%282023%29.%20Multifaceted%20modes%20of%20%26%23x3B3%3B-tubulin%20complex%20recruitment%20and%20microtubule%20nucleation%20at%20mitotic%20centrosomes.%20%26lt%3Bi%26gt%3BJournal%20of%20Cell%20Biology%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B222%26lt%3B%5C%2Fi%26gt%3B%2810%29%2C%20e202212043.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1083%5C%2Fjcb.202212043%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1083%5C%2Fjcb.202212043%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Multifaceted%20modes%20of%20%5Cu03b3-tubulin%20complex%20recruitment%20and%20microtubule%20nucleation%20at%20mitotic%20centrosomes%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Zihan%22%2C%22lastName%22%3A%22Zhu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Isabelle%22%2C%22lastName%22%3A%22Becam%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Corinne%20A.%22%2C%22lastName%22%3A%22Tovey%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Abir%22%2C%22lastName%22%3A%22Elfarkouchi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Eugenie%20C.%22%2C%22lastName%22%3A%22Yen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fred%22%2C%22lastName%22%3A%22Bernard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Paul%20T.%22%2C%22lastName%22%3A%22Conduit%22%7D%5D%2C%22abstractNote%22%3A%22Microtubule%20nucleation%20is%20mediated%20by%20%5Cu03b3-tubulin%20ring%20complexes%20%28%5Cu03b3-TuRCs%29.%20In%20most%20eukaryotes%2C%20a%20GCP4%5C%2F5%5C%2F4%5C%2F6%20%5Cu201ccore%5Cu201d%20complex%20promotes%20%5Cu03b3-tubulin%20small%20complex%20%28%5Cu03b3-TuSC%29%20association%20to%20generate%20cytosolic%20%5Cu03b3-TuRCs.%20Unlike%20%5Cu03b3-TuSCs%2C%20however%2C%20this%20core%20complex%20is%20non-essential%20in%20various%20species%20and%20absent%20from%20budding%20yeasts.%20In%20Drosophila%2C%20Spindle%20defective-2%20%28Spd-2%29%20and%20Centrosomin%20%28Cnn%29%20redundantly%20recruit%20%5Cu03b3-tubulin%20complexes%20to%20mitotic%20centrosomes.%20Here%2C%20we%20show%20that%20Spd-2%20recruits%20%5Cu03b3-TuRCs%20formed%20via%20the%20GCP4%5C%2F5%5C%2F4%5C%2F6%20core%2C%20but%20Cnn%20can%20recruit%20%5Cu03b3-TuSCs%20directly%20via%20its%20well-conserved%20CM1%20domain%2C%20similar%20to%20its%20homologs%20in%20budding%20yeast.%20When%20centrosomes%20fail%20to%20recruit%20%5Cu03b3-tubulin%20complexes%2C%20they%20still%20nucleate%20microtubules%20via%20the%20TOG%20domain%20protein%20Mini-spindles%20%28Msps%29%2C%20but%20these%20microtubules%20have%20different%20dynamic%20properties.%20Our%20data%2C%20therefore%2C%20help%20explain%20the%20dispensability%20of%20the%20GCP4%5C%2F5%5C%2F4%5C%2F6%20core%20and%20highlight%20the%20robustness%20of%20centrosomes%20as%20microtubule%20organizing%20centers.%20They%20also%20suggest%20that%20the%20dynamic%20properties%20of%20microtubules%20are%20influenced%20by%20how%20they%20are%20nucleated.%22%2C%22date%22%3A%222023-09-12%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1083%5C%2Fjcb.202212043%22%2C%22ISSN%22%3A%220021-9525%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1083%5C%2Fjcb.202212043%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%2C%227DMXI4X8%22%5D%2C%22dateModified%22%3A%222023-09-22T10%3A25%3A35Z%22%7D%7D%2C%7B%22key%22%3A%22NUNDKNSV%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Loh%20et%20al.%22%2C%22parsedDate%22%3A%222023-06-19%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BLoh%2C%20M.%2C%20Bernard%2C%20F.%2C%20%26amp%3B%20Guichet%2C%20A.%20%282023%29.%20Kinesin-1%20promotes%20centrosome%20clustering%20and%20nuclear%20migration%20in%20the%20Drosophila%20oocyte.%20%26lt%3Bi%26gt%3BDevelopment%26lt%3B%5C%2Fi%26gt%3B%2C%20dev.201728.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fdev.201728%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fdev.201728%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Kinesin-1%20promotes%20centrosome%20clustering%20and%20nuclear%20migration%20in%20the%20Drosophila%20oocyte%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ma%5Cu00eblys%22%2C%22lastName%22%3A%22Loh%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fred%22%2C%22lastName%22%3A%22Bernard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%5D%2C%22abstractNote%22%3A%22Microtubules%20and%20their%20associated%20motors%20are%20important%20players%20in%20nucleus%20positioning.%20Although%20nuclear%20migration%20in%20Drosophila%20oocytes%20is%20controlled%20by%20microtubules%2C%20a%20precise%20role%20for%20microtubule-associated%20molecular%20motors%20in%20nuclear%20migration%20has%20yet%20to%20be%20reported.%20We%20characterize%20novel%20landmarks%20that%20allow%20a%20precise%20description%20of%20the%20pre-migratory%20stages.%20Using%20these%20newly%20defined%20stages%2C%20we%20report%20that%2C%20prior%20to%20migration%2C%20the%20nucleus%20moves%20from%20the%20oocyte%20anterior%20side%20toward%20the%20center%20and%20concomitantly%20the%20centrosomes%20cluster%20at%20the%20posterior%20of%20the%20nucleus.%20In%20absence%20of%20Kinesin-1%2C%20centrosome%20clustering%20is%20impaired%20and%20the%20nucleus%20fails%20to%20position%20and%20migrate%20properly.%20The%20maintenance%20of%20a%20high%20level%20of%20Polo-kinase%20at%20centrosomes%20prevents%20centrosome%20clustering%20and%20impairs%20nuclear%20positioning.%20In%20absence%20of%20Kinesin-1%2C%20SPD-2%20an%20essential%20component%20of%20the%20pericentriolar%20material%20is%20increased%20at%20the%20centrosomes%2C%20suggesting%20that%20Kinesin-1%20associated%20defects%20result%20from%20a%20failure%20to%20reduce%20centrosome%20activity.%20Consistently%2C%20depleting%20centrosomes%20rescues%20the%20nuclear%20migration%20defects%20induced%20by%20Kinesin-1%20inactivation.%20Our%20results%20suggest%20that%20Kinesin-1%20controls%20nuclear%20migration%20in%20the%20oocyte%20by%20modulating%20centrosome%20activity.%22%2C%22date%22%3A%222023-06-19%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1242%5C%2Fdev.201728%22%2C%22ISSN%22%3A%220950-1991%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fdev.201728%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222023-06-19T12%3A03%3A38Z%22%7D%7D%2C%7B%22key%22%3A%22I3LH3KBZ%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Galenza%20et%20al.%22%2C%22parsedDate%22%3A%222023-03-30%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BGalenza%2C%20A.%2C%20Moreno-Roman%2C%20P.%2C%20Su%2C%20Y.-H.%2C%20Acosta-Alvarez%2C%20L.%2C%20Debec%2C%20A.%2C%20Guichet%2C%20A.%2C%20Knapp%2C%20J.-M.%2C%20Kizilyaprak%2C%20C.%2C%20Humbel%2C%20B.%20M.%2C%20Kolotuev%2C%20I.%2C%20%26amp%3B%20O%26%23×2019%3BBrien%2C%20L.%20E.%20%282023%29.%20Basal%20stem%20cell%20progeny%20establish%20their%20apical%20surface%20in%20a%20junctional%20niche%20during%20turnover%20of%20an%20adult%20barrier%20epithelium.%20%26lt%3Bi%26gt%3BNature%20Cell%20Biology%26lt%3B%5C%2Fi%26gt%3B.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1038%5C%2Fs41556-023-01116-w%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1038%5C%2Fs41556-023-01116-w%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Basal%20stem%20cell%20progeny%20establish%20their%20apical%20surface%20in%20a%20junctional%20niche%20during%20turnover%20of%20an%20adult%20barrier%20epithelium%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anthony%22%2C%22lastName%22%3A%22Galenza%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Paola%22%2C%22lastName%22%3A%22Moreno-Roman%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yu-Han%22%2C%22lastName%22%3A%22Su%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Lehi%22%2C%22lastName%22%3A%22Acosta-Alvarez%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Alain%22%2C%22lastName%22%3A%22Debec%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jon-Michael%22%2C%22lastName%22%3A%22Knapp%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Caroline%22%2C%22lastName%22%3A%22Kizilyaprak%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Bruno%20M.%22%2C%22lastName%22%3A%22Humbel%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Irina%22%2C%22lastName%22%3A%22Kolotuev%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Lucy%20Erin%22%2C%22lastName%22%3A%22O%27Brien%22%7D%5D%2C%22abstractNote%22%3A%22Barrier%20epithelial%20organs%20face%20the%20constant%20challenge%20of%20sealing%20the%20interior%20body%20from%20the%20external%20environment%20while%20simultaneously%20replacing%20the%20cells%20that%20contact%20this%20environment.%20New%20replacement%20cells-the%20progeny%20of%20basal%20stem%20cells-are%20born%20without%20barrier-forming%20structures%20such%20as%20a%20specialized%20apical%20membrane%20and%20occluding%20junctions.%20Here%2C%20we%20investigate%20how%20new%20progeny%20acquire%20barrier%20structures%20as%20they%20integrate%20into%20the%20intestinal%20epithelium%20of%20adult%20Drosophila.%20We%20find%20they%20gestate%20their%20future%20apical%20membrane%20in%20a%20sublumenal%20niche%20created%20by%20a%20transitional%20occluding%20junction%20that%20envelops%20the%20differentiating%20cell%20and%20enables%20it%20to%20form%20a%20deep%2C%20microvilli-lined%20apical%20pit.%20The%20transitional%20junction%20seals%20the%20pit%20from%20the%20intestinal%20lumen%20until%20differentiation-driven%2C%20basal-to-apical%20remodelling%20of%20the%20niche%20opens%20the%20pit%20and%20integrates%20the%20now-mature%20cell%20into%20the%20barrier.%20By%20coordinating%20junctional%20remodelling%20with%20terminal%20differentiation%2C%20stem%20cell%20progeny%20integrate%20into%20a%20functional%2C%20adult%20epithelium%20without%20jeopardizing%20barrier%20integrity.%22%2C%22date%22%3A%222023-03-30%22%2C%22language%22%3A%22eng%22%2C%22DOI%22%3A%2210.1038%5C%2Fs41556-023-01116-w%22%2C%22ISSN%22%3A%221476-4679%22%2C%22url%22%3A%22%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222023-05-03T12%3A57%3A20Z%22%7D%7D%2C%7B%22key%22%3A%224ZIS5J5L%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Tovey%20et%20al.%22%2C%22parsedDate%22%3A%222021-08-02%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BTovey%2C%20C.%20A.%2C%20Tsuji%2C%20C.%2C%20Egerton%2C%20A.%2C%20Bernard%2C%20F.%2C%20Guichet%2C%20A.%2C%20de%20la%20Roche%2C%20M.%2C%20%26amp%3B%20Conduit%2C%20P.%20T.%20%282021%29.%20Autoinhibition%20of%20Cnn%20binding%20to%20%26%23x3B3%3B-TuRCs%20prevents%20ectopic%20microtubule%20nucleation%20and%20cell%20division%20defects.%20%26lt%3Bi%26gt%3BThe%20Journal%20of%20Cell%20Biology%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B220%26lt%3B%5C%2Fi%26gt%3B%288%29%2C%20e202010020.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1083%5C%2Fjcb.202010020%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1083%5C%2Fjcb.202010020%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Autoinhibition%20of%20Cnn%20binding%20to%20%5Cu03b3-TuRCs%20prevents%20ectopic%20microtubule%20nucleation%20and%20cell%20division%20defects%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Corinne%20A.%22%2C%22lastName%22%3A%22Tovey%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chisato%22%2C%22lastName%22%3A%22Tsuji%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Alice%22%2C%22lastName%22%3A%22Egerton%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fred%22%2C%22lastName%22%3A%22Bernard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Marc%22%2C%22lastName%22%3A%22de%20la%20Roche%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Paul%20T.%22%2C%22lastName%22%3A%22Conduit%22%7D%5D%2C%22abstractNote%22%3A%22%5Cu03b3-Tubulin%20ring%20complexes%20%28%5Cu03b3-TuRCs%29%20nucleate%20microtubules.%20They%20are%20recruited%20to%20centrosomes%20in%20dividing%20cells%20via%20binding%20to%20N-terminal%20CM1%20domains%20within%20%5Cu03b3-TuRC-tethering%20proteins%2C%20including%20Drosophila%20Centrosomin%20%28Cnn%29.%20Binding%20promotes%20microtubule%20nucleation%20and%20is%20restricted%20to%20centrosomes%20in%20dividing%20cells%2C%20but%20the%20mechanism%20regulating%20binding%20remains%20unknown.%20Here%2C%20we%20identify%20an%20extreme%20N-terminal%20CM1%20autoinhibition%20%28CAI%29%20domain%20found%20specifically%20within%20the%20centrosomal%20isoform%20of%20Cnn%20%28Cnn-C%29%20that%20inhibits%20%5Cu03b3-TuRC%20binding.%20Robust%20binding%20occurs%20after%20removal%20of%20the%20CAI%20domain%20or%20with%20the%20addition%20of%20phosphomimetic%20mutations%2C%20suggesting%20that%20phosphorylation%20helps%20relieve%20inhibition.%20We%20show%20that%20regulation%20of%20Cnn%20binding%20to%20%5Cu03b3-TuRCs%20is%20isoform%20specific%20and%20that%20misregulation%20of%20binding%20can%20result%20in%20ectopic%20cytosolic%20microtubules%20and%20major%20defects%20during%20cell%20division.%20We%20also%20find%20that%20human%20CDK5RAP2%20is%20autoinhibited%20from%20binding%20%5Cu03b3-TuRCs%2C%20suggesting%20conservation%20across%20species.%20Overall%2C%20our%20results%20shed%20light%20on%20how%20and%20why%20CM1%20domain%20binding%20to%20%5Cu03b3-TuRCs%20is%20regulated.%22%2C%22date%22%3A%222021-08-02%22%2C%22language%22%3A%22eng%22%2C%22DOI%22%3A%2210.1083%5C%2Fjcb.202010020%22%2C%22ISSN%22%3A%221540-8140%22%2C%22url%22%3A%22%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222023-05-02T12%3A16%3A48Z%22%7D%7D%2C%7B%22key%22%3A%226L56GKM8%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Loh%20et%20al.%22%2C%22parsedDate%22%3A%222021-05-13%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BLoh%2C%20M.%2C%20Guichet%2C%20A.%2C%20%26amp%3B%20Bernard%2C%20F.%20%282021%29.%20Nuclear%20Migration%20in%20the%20Drosophila%20Oocyte.%20%26lt%3Bi%26gt%3BJoVE%20%28Journal%20of%20Visualized%20Experiments%29%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B171%26lt%3B%5C%2Fi%26gt%3B%2C%20e62688.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3791%5C%2F62688%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3791%5C%2F62688%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Nuclear%20Migration%20in%20the%20Drosophila%20Oocyte%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ma%5Cu00eblys%22%2C%22lastName%22%3A%22Loh%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fred%22%2C%22lastName%22%3A%22Bernard%22%7D%5D%2C%22abstractNote%22%3A%22Live%20cell%20imaging%20is%20particularly%20necessary%20to%20understand%20the%20cellular%20and%20molecular%20mechanisms%20that%20regulate%20organelle%20movements%2C%20cytoskeleton%20rearrangements%2C%20or%20polarity%20patterning%20within%20the%20cells.%20When%20studying%20oocyte%20nucleus%20positioning%2C%20live-imaging%20techniques%20are%20essential%20to%20capture%20the%20dynamic%20events%20of%20this%20process.%20The%20Drosophila%20egg%20chamber%20is%20a%20multicellular%20structure%20and%20an%20excellent%20model%20system%20to%20study%20this%20phenomenon%20because%20of%20its%20large%20size%20and%20availability%20of%20numerous%20genetic%20tools.%20During%20Drosophila%20mid-oogenesis%2C%20the%20nucleus%20migrates%20from%20a%20central%20position%20within%20the%20oocyte%20to%20adopt%20an%20asymmetric%20position%20mediated%20by%20microtubule-generated%20forces.%20This%20migration%20and%20positioning%20of%20the%20nucleus%20are%20necessary%20to%20determine%20the%20polarity%20axes%20of%20the%20embryo%20and%20the%20subsequent%20adult%20fly.%20One%20characteristic%20of%20this%20migration%20is%20that%20it%20occurs%20in%20three%20dimensions%20%283D%29%2C%20creating%20a%20necessity%20for%20live%20imaging.%20Thus%2C%20to%20study%20the%20mechanisms%20that%20regulate%20nuclear%20migration%2C%20we%20have%20developed%20a%20protocol%20to%20culture%20the%20dissected%20egg%20chambers%20and%20perform%20live%20imaging%20for%2012%20h%20by%20time-lapse%20acquisitions%20using%20spinning-disk%20confocal%20microscopy.%20Overall%2C%20our%20conditions%20allow%20us%20to%20preserve%20Drosophila%20egg%20chambers%20alive%20for%20a%20long%20period%20of%20time%2C%20thereby%20enabling%20the%20completion%20of%20nuclear%20migration%20to%20be%20visualized%20in%20a%20large%20number%20of%20samples%20in%203D.%22%2C%22date%22%3A%222021%5C%2F05%5C%2F13%22%2C%22language%22%3A%22fr%22%2C%22DOI%22%3A%2210.3791%5C%2F62688%22%2C%22ISSN%22%3A%221940-087X%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.jove.com%5C%2Ffr%5C%2Fv%5C%2F62688%5C%2Fnuclear-migration-in-the-drosophila-oocyte%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A04%3A12Z%22%7D%7D%2C%7B%22key%22%3A%22WWC4EQ7Y%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Bernard%20et%20al.%22%2C%22parsedDate%22%3A%222021%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BBernard%2C%20F.%2C%20Jouette%2C%20J.%2C%20Durieu%2C%20C.%2C%20Le%20Borgne%2C%20R.%2C%20Guichet%2C%20A.%2C%20%26amp%3B%20Claret%2C%20S.%20%282021%29.%20GFP-Tagged%20Protein%20Detection%20by%20Electron%20Microscopy%20Using%20a%20GBP-APEX%20Tool%20in%20Drosophila.%20%26lt%3Bi%26gt%3BFrontiers%20in%20Cell%20and%20Developmental%20Biology%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B9%26lt%3B%5C%2Fi%26gt%3B.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fwww.frontiersin.org%5C%2Farticles%5C%2F10.3389%5C%2Ffcell.2021.719582%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fwww.frontiersin.org%5C%2Farticles%5C%2F10.3389%5C%2Ffcell.2021.719582%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22GFP-Tagged%20Protein%20Detection%20by%20Electron%20Microscopy%20Using%20a%20GBP-APEX%20Tool%20in%20Drosophila%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fred%22%2C%22lastName%22%3A%22Bernard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Julie%22%2C%22lastName%22%3A%22Jouette%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Catherine%22%2C%22lastName%22%3A%22Durieu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22R%5Cu00e9mi%22%2C%22lastName%22%3A%22Le%20Borgne%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sandra%22%2C%22lastName%22%3A%22Claret%22%7D%5D%2C%22abstractNote%22%3A%22In%20cell%20biology%2C%20detection%20of%20protein%20subcellular%20localizations%20is%20often%20achieved%20by%20optical%20microscopy%20techniques%20and%20more%20rarely%20by%20electron%20microscopy%20%28EM%29%20despite%20the%20greater%20resolution%20offered%20by%20EM.%20One%20of%20the%20possible%20reasons%20was%20that%20protein%20detection%20by%20EM%20required%20specific%20antibodies%20whereas%20this%20need%20could%20be%20circumvented%20by%20using%20fluorescently-tagged%20proteins%20in%20optical%20microscopy%20approaches.%20Recently%2C%20the%20description%20of%20a%20genetically%20encodable%20EM%20tag%2C%20the%20engineered%20ascorbate%20peroxidase%20%28APEX%29%2C%20whose%20activity%20can%20be%20monitored%20by%20electron-dense%20DAB%20precipitates%2C%20has%20widened%20the%20possibilities%20of%20specific%20protein%20detection%20in%20EM.%20However%2C%20this%20technique%20still%20requires%20the%20generation%20of%20new%20molecular%20constructions.%20Thus%2C%20we%20decided%20to%20develop%20a%20versatile%20method%20that%20would%20take%20advantage%20of%20the%20numerous%20GFP-tagged%20proteins%20already%20existing%20and%20create%20a%20tool%20combining%20a%20nanobody%20anti-GFP%20%28GBP%29%20with%20APEX.%20This%20GBP-APEX%20tool%20allows%20a%20simple%20and%20efficient%20detection%20of%20any%20GFP%20fusion%20proteins%20without%20the%20needs%20of%20specific%20antibodies%20nor%20the%20generation%20of%20additional%20constructions.%20We%20have%20shown%20the%20feasibility%20and%20efficiency%20of%20this%20method%20to%20detect%20various%20proteins%20in%20Drosophila%20ovarian%20follicles%20such%20as%20nuclear%20proteins%2C%20proteins%20associated%20with%20endocytic%20vesicles%2C%20plasma%20membranes%20or%20nuclear%20envelopes.%20Lastly%2C%20we%20expressed%20this%20tool%20in%20Drosophila%20with%20the%20UAS%5C%2FGAL4%20system%20that%20enables%20spatiotemporal%20control%20of%20the%20protein%20detection.%22%2C%22date%22%3A%222021%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22ISSN%22%3A%222296-634X%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.frontiersin.org%5C%2Farticles%5C%2F10.3389%5C%2Ffcell.2021.719582%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222023-06-06T09%3A42%3A04Z%22%7D%7D%2C%7B%22key%22%3A%22EYK6FGWU%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Mukherjee%20et%20al.%22%2C%22parsedDate%22%3A%222020-07-13%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BMukherjee%2C%20A.%2C%20Brooks%2C%20P.%20S.%2C%20Bernard%2C%20F.%2C%20Guichet%2C%20A.%2C%20%26amp%3B%20Conduit%2C%20P.%20T.%20%282020%29.%20Microtubules%20originate%20asymmetrically%20at%20the%20somatic%20golgi%20and%20are%20guided%20via%20Kinesin2%20to%20maintain%20polarity%20within%20neurons.%20%26lt%3Bi%26gt%3BELife%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B9%26lt%3B%5C%2Fi%26gt%3B%2C%20e58943.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.7554%5C%2FeLife.58943%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.7554%5C%2FeLife.58943%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Microtubules%20originate%20asymmetrically%20at%20the%20somatic%20golgi%20and%20are%20guided%20via%20Kinesin2%20to%20maintain%20polarity%20within%20neurons%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Amrita%22%2C%22lastName%22%3A%22Mukherjee%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Paul%20S.%22%2C%22lastName%22%3A%22Brooks%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fred%22%2C%22lastName%22%3A%22Bernard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Paul%20T.%22%2C%22lastName%22%3A%22Conduit%22%7D%5D%2C%22abstractNote%22%3A%22Neurons%20contain%20polarised%20microtubule%20arrays%20essential%20for%20neuronal%20function.%20How%20microtubule%20nucleation%20and%20polarity%20are%20regulated%20within%20neurons%20remains%20unclear.%20We%20show%20that%20%5Cu03b3-tubulin%20localises%20asymmetrically%20to%20the%20somatic%20Golgi%20within%20Drosophila%20neurons.%20Microtubules%20originate%20from%20the%20Golgi%20with%20an%20initial%20growth%20preference%20towards%20the%20axon.%20Their%20growing%20plus%20ends%20also%20turn%20towards%20and%20into%20the%20axon%2C%20adding%20to%20the%20plus-end-out%20microtubule%20pool.%20Any%20plus%20ends%20that%20reach%20a%20dendrite%2C%20however%2C%20do%20not%20readily%20enter%2C%20maintaining%20minus-end-out%20polarity.%20Both%20turning%20towards%20the%20axon%20and%20exclusion%20from%20dendrites%20depend%20on%20Kinesin-2%2C%20a%20plus-end-associated%20motor%20that%20guides%20growing%20plus%20ends%20along%20adjacent%20microtubules.%20We%20propose%20that%20Kinesin-2%20engages%20with%20a%20polarised%20microtubule%20network%20within%20the%20soma%20to%20guide%20growing%20microtubules%20towards%20the%20axon%3B%20while%20at%20dendrite%20entry%20sites%20engagement%20with%20microtubules%20of%20opposite%20polarity%20generates%20a%20backward%20stalling%20force%20that%20prevents%20entry%20into%20dendrites%20and%20thus%20maintains%20minus-end-out%20polarity%20within%20proximal%20dendrites.%22%2C%22date%22%3A%222020-07-13%22%2C%22language%22%3A%22eng%22%2C%22DOI%22%3A%2210.7554%5C%2FeLife.58943%22%2C%22ISSN%22%3A%222050-084X%22%2C%22url%22%3A%22%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222023-05-02T13%3A35%3A24Z%22%7D%7D%2C%7B%22key%22%3A%22W5I6NMFD%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22lastModifiedByUser%22%3A%7B%22id%22%3A11274337%2C%22username%22%3A%22Charlotte_Brancaz%22%2C%22name%22%3A%22%22%2C%22links%22%3A%7B%22alternate%22%3A%7B%22href%22%3A%22https%3A%5C%2F%5C%2Fwww.zotero.org%5C%2Fcharlotte_brancaz%22%2C%22type%22%3A%22text%5C%2Fhtml%22%7D%7D%7D%2C%22creatorSummary%22%3A%22M%5Cu00e9tivier%20et%20al.%22%2C%22parsedDate%22%3A%222019-04-17%22%2C%22numChildren%22%3A3%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BM%26%23xE9%3Btivier%2C%20M.%2C%20Monroy%2C%20B.%20Y.%2C%20Gallaud%2C%20E.%2C%20Caous%2C%20R.%2C%20Pascal%2C%20A.%2C%20Richard-Parpaillon%2C%20L.%2C%20Guichet%2C%20A.%2C%20Ori-McKenney%2C%20K.%20M.%2C%20%26amp%3B%20Giet%2C%20R.%20%282019%29.%20Dual%20control%20of%20Kinesin-1%20recruitment%20to%20microtubules%20by%20Ensconsin%20in%20Drosophila%20neuroblasts%20and%20oocytes.%20%26lt%3Bi%26gt%3BDevelopment%20%28Cambridge%2C%20England%29%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B146%26lt%3B%5C%2Fi%26gt%3B%288%29%2C%20dev171579.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fdev.171579%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fdev.171579%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Dual%20control%20of%20Kinesin-1%20recruitment%20to%20microtubules%20by%20Ensconsin%20in%20Drosophila%20neuroblasts%20and%20oocytes%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mathieu%22%2C%22lastName%22%3A%22M%5Cu00e9tivier%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Brigette%20Y.%22%2C%22lastName%22%3A%22Monroy%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Emmanuel%22%2C%22lastName%22%3A%22Gallaud%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Renaud%22%2C%22lastName%22%3A%22Caous%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Aude%22%2C%22lastName%22%3A%22Pascal%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Laurent%22%2C%22lastName%22%3A%22Richard-Parpaillon%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kassandra%20M.%22%2C%22lastName%22%3A%22Ori-McKenney%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22R%5Cu00e9gis%22%2C%22lastName%22%3A%22Giet%22%7D%5D%2C%22abstractNote%22%3A%22Drosophila%20Ensconsin%20%28also%20known%20as%20MAP7%29%20controls%20spindle%20length%2C%20centrosome%20separation%20in%20brain%20neuroblasts%20%28NBs%29%20and%20asymmetric%20transport%20in%20oocytes.%20The%20control%20of%20spindle%20length%20by%20Ensconsin%20is%20Kinesin-1%20independent%20but%20centrosome%20separation%20and%20oocyte%20transport%20require%20targeting%20of%20Kinesin-1%20to%20microtubules%20by%20Ensconsin.%20However%2C%20the%20molecular%20mechanism%20used%20for%20this%20targeting%20remains%20unclear.%20Ensconsin%20contains%20a%20microtubule%20%28MT%29-binding%20domain%20%28MBD%29%20and%20a%20Kinesin-binding%20domain%20%28KBD%29.%20Rescue%20experiments%20show%20that%20only%20full-length%20Ensconsin%20restores%20the%20spindle%20length%20phenotype.%20KBD%20expression%20rescues%20ensc%20centrosome%20separation%20defects%20in%20NBs%2C%20but%20not%20the%20fast%20oocyte%20streaming%20and%20the%20localization%20of%20Staufen%20and%20Gurken.%20Interestingly%2C%20the%20KBD%20can%20stimulate%20Kinesin-1%20targeting%20to%20MTs%20in%20vivo%20and%20in%20vitro%20We%20propose%20that%20a%20KBD%20and%20Kinesin-1%20complex%20is%20a%20minimal%20activation%20module%20that%20increases%20Kinesin-1%20affinity%20for%20MTs.%20Addition%20of%20the%20MBD%20present%20in%20full-length%20Ensconsin%20allows%20this%20process%20to%20occur%20directly%20on%20the%20MT%20and%20triggers%20higher%20Kinesin-1%20targeting.%20This%20dual%20regulation%20by%20Ensconsin%20is%20essential%20for%20optimal%20Kinesin-1%20targeting%20to%20MTs%20in%20oocytes%2C%20but%20not%20in%20NBs%2C%20illustrating%20the%20importance%20of%20adapting%20Kinesin-1%20recruitment%20to%20different%20biological%20contexts.%22%2C%22date%22%3A%222019-04-17%22%2C%22language%22%3A%22eng%22%2C%22DOI%22%3A%2210.1242%5C%2Fdev.171579%22%2C%22ISSN%22%3A%221477-9129%22%2C%22url%22%3A%22%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222022-09-05T13%3A27%3A23Z%22%7D%7D%2C%7B%22key%22%3A%22Z8L98CQZ%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Jouette%20et%20al.%22%2C%22parsedDate%22%3A%222019-01-23%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BJouette%2C%20J.%2C%20Guichet%2C%20A.%2C%20%26amp%3B%20Claret%2C%20S.%20B.%20%282019%29.%20Dynein-mediated%20transport%20and%20membrane%20trafficking%20control%20PAR3%20polarised%20distribution.%20%26lt%3Bi%26gt%3BELife%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B8%26lt%3B%5C%2Fi%26gt%3B%2C%20e40212.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.7554%5C%2FeLife.40212%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.7554%5C%2FeLife.40212%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Dynein-mediated%20transport%20and%20membrane%20trafficking%20control%20PAR3%20polarised%20distribution%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Julie%22%2C%22lastName%22%3A%22Jouette%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sandra%20B.%22%2C%22lastName%22%3A%22Claret%22%7D%5D%2C%22abstractNote%22%3A%22The%20scaffold%20protein%20PAR3%20and%20the%20kinase%20PAR1%20are%20essential%20proteins%20that%20control%20cell%20polarity.%20Their%20precise%20opposite%20localisations%20define%20plasma%20membrane%20domains%20with%20specific%20functions.%20PAR3%20and%20PAR1%20are%20mutually%20inhibited%20by%20direct%20or%20indirect%20phosphorylations%2C%20but%20their%20fates%20once%20phosphorylated%20are%20poorly%20known.%20Through%20precise%20spatiotemporal%20quantification%20of%20PAR3%20localisation%20in%20the%20Drosophila%20oocyte%2C%20we%20identify%20several%20mechanisms%20responsible%20for%20its%20anterior%20cortex%20accumulation%20and%20its%20posterior%20exclusion.%20We%20show%20that%20PAR3%20posterior%20plasma%20membrane%20exclusion%20depends%20on%20PAR1%20and%20an%20endocytic%20mechanism%20relying%20on%20RAB5%20and%20PI%284%2C5%29P2.%20In%20a%20second%20phase%2C%20microtubules%20and%20the%20dynein%20motor%2C%20in%20connection%20with%20vesicular%20trafficking%20involving%20RAB11%20and%20IKK-related%20kinase%2C%20IKK%5Cu03b5%2C%20are%20required%20for%20PAR3%20transport%20towards%20the%20anterior%20cortex.%20Altogether%2C%20our%20results%20point%20to%20a%20connection%20between%20membrane%20trafficking%20and%20dynein-mediated%20transport%20to%20sustain%20PAR3%20asymmetry.%22%2C%22date%22%3A%222019-01-23%22%2C%22language%22%3A%22eng%22%2C%22DOI%22%3A%2210.7554%5C%2FeLife.40212%22%2C%22ISSN%22%3A%222050-084X%22%2C%22url%22%3A%22%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222023-05-02T14%3A24%3A59Z%22%7D%7D%2C%7B%22key%22%3A%22F6EHQTLE%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22lastModifiedByUser%22%3A%7B%22id%22%3A11274337%2C%22username%22%3A%22Charlotte_Brancaz%22%2C%22name%22%3A%22%22%2C%22links%22%3A%7B%22alternate%22%3A%7B%22href%22%3A%22https%3A%5C%2F%5C%2Fwww.zotero.org%5C%2Fcharlotte_brancaz%22%2C%22type%22%3A%22text%5C%2Fhtml%22%7D%7D%7D%2C%22creatorSummary%22%3A%22Bernard%20et%20al.%22%2C%22parsedDate%22%3A%222018-10%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BBernard%2C%20F.%2C%20Lepesant%2C%20J.-A.%2C%20%26amp%3B%20Guichet%2C%20A.%20%282018%29.%20Nucleus%20positioning%20within%20Drosophila%20egg%20chamber.%20%26lt%3Bi%26gt%3BSeminars%20in%20Cell%20%26amp%3B%20Developmental%20Biology%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B82%26lt%3B%5C%2Fi%26gt%3B%2C%2025%26%23×2013%3B33.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.semcdb.2017.10.013%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.semcdb.2017.10.013%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Nucleus%20positioning%20within%20Drosophila%20egg%20chamber%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fred%22%2C%22lastName%22%3A%22Bernard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jean-Antoine%22%2C%22lastName%22%3A%22Lepesant%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%5D%2C%22abstractNote%22%3A%22Both%20types%20of%20Drosophila%20egg%20chamber%20germ%20cells%2C%20i.e.%20oocyte%20and%20nurse%20cells%2C%20have%20to%20control%20their%20nucleus%20positions%20in%20order%20to%20produce%20a%20viable%20gamete.%20Interestingly%2C%20while%20actin%20microfilaments%20are%20crucial%20to%20position%20the%20nuclei%20in%20nurse%20cells%2C%20these%20are%20the%20microtubules%20that%20are%20important%20for%20oocyte%20nucleus%20to%20migrate%20and%20adopt%20the%20correct%20position.%20In%20this%20review%2C%20we%20discuss%20the%20mechanisms%20underlying%20these%20positioning%20processes%20in%20the%20two%20cell%20types%20with%20respect%20to%20the%20organization%20and%20dynamics%20of%20the%20actin%20and%20microtubule%20skeleton.%20In%20the%20nurse%20cells%20it%20is%20essential%20to%20keep%20firmly%20the%20nuclei%20in%20a%20central%20position%20to%20prevent%20them%20from%20obstructing%20the%20ring%20canals%20when%20the%20cytoplasmic%20content%20of%20the%20cells%20is%20dumped%20into%20the%20oocyte%20cells%20toward%20the%20end%20of%20oogenesis.%20This%20is%20achieved%20by%20the%20assembly%20of%20thick%20filopodia-like%20actin%20cables%20anchored%20to%20the%20plasma%20membrane%2C%20which%20grow%20inwardly%20and%20eventually%20encase%20tightly%20the%20nuclei%20in%20a%20cage-like%20structure.%20In%20the%20oocyte%2C%20the%20migration%20at%20an%20early%20stage%20of%20oogenesis%20of%20the%20nucleus%20from%20a%20posterior%20location%20to%20an%20anchorage%20site%20at%20an%20asymmetric%20anterior%20position%2C%20is%20an%20essential%20step%20in%20the%20setting%20up%20of%20the%20dorsoventral%20polarity%20axis%20of%20the%20future%20embryo.%20This%20process%20is%20controlled%20by%20an%20interplay%20between%20MT%20networks%20that%20just%20start%20to%20be%20untangled.%20Although%20both%20mechanisms%20have%20evolved%20to%20fulfill%20cell-type%20specific%20cell%20processes%20in%20the%20context%20of%20fly%20oogenesis%2C%20interesting%20parallels%20can%20be%20drawn%20with%20other%20nuclear%20positioning%20mechanisms%20in%20the%20mouse%20oocyte%20and%20the%20developing%20muscle%20respectively.%22%2C%22date%22%3A%222018-10%22%2C%22language%22%3A%22eng%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.semcdb.2017.10.013%22%2C%22ISSN%22%3A%221096-3634%22%2C%22url%22%3A%22%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222022-09-05T13%3A28%3A03Z%22%7D%7D%2C%7B%22key%22%3A%22A5BUM8LT%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Tissot%20et%20al.%22%2C%22parsedDate%22%3A%222017-04-27%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BTissot%2C%20N.%2C%20Lepesant%2C%20J.-A.%2C%20Bernard%2C%20F.%2C%20Legent%2C%20K.%2C%20Bosveld%2C%20F.%2C%20Martin%2C%20C.%2C%20Faklaris%2C%20O.%2C%20Bella%26%23xEF%3Bche%2C%20Y.%2C%20Coppey%2C%20M.%2C%20%26amp%3B%20Guichet%2C%20A.%20%282017%29.%20Distinct%20molecular%20cues%20ensure%20a%20robust%20microtubule-dependent%20nuclear%20positioning%20in%20the%20Drosophila%20oocyte.%20%26lt%3Bi%26gt%3BNature%20Communications%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B8%26lt%3B%5C%2Fi%26gt%3B%281%29%2C%2015168.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1038%5C%2Fncomms15168%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1038%5C%2Fncomms15168%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Distinct%20molecular%20cues%20ensure%20a%20robust%20microtubule-dependent%20nuclear%20positioning%20in%20the%20Drosophila%20oocyte%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nicolas%22%2C%22lastName%22%3A%22Tissot%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jean-Antoine%22%2C%22lastName%22%3A%22Lepesant%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fred%22%2C%22lastName%22%3A%22Bernard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kevin%22%2C%22lastName%22%3A%22Legent%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Floris%22%2C%22lastName%22%3A%22Bosveld%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Charlotte%22%2C%22lastName%22%3A%22Martin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Orestis%22%2C%22lastName%22%3A%22Faklaris%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yohanns%22%2C%22lastName%22%3A%22Bella%5Cu00efche%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ma%5Cu00eft%5Cu00e9%22%2C%22lastName%22%3A%22Coppey%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%5D%2C%22abstractNote%22%3A%22Controlling%20nucleus%20localization%20is%20crucial%20for%20a%20variety%20of%20cellular%20functions.%20In%20the%20Drosophila%20oocyte%2C%20nuclear%20asymmetric%20positioning%20is%20essential%20for%20the%20reorganization%20of%20the%20microtubule%20%28MT%29%20network%20that%20controls%20the%20polarized%20transport%20of%20axis%20determinants.%20A%20combination%20of%20quantitative%20three-dimensional%20live%20imaging%20and%20laser%20ablation-mediated%20force%20analysis%20reveal%20that%20nuclear%20positioning%20is%20ensured%20with%20an%20unexpected%20level%20of%20robustness.%20We%20show%20that%20the%20nucleus%20is%20pushed%20to%20the%20oocyte%20antero-dorsal%20cortex%20by%20MTs%20and%20that%20its%20migration%20can%20proceed%20through%20distinct%20tracks.%20Centrosome-associated%20MTs%20favour%20one%20migratory%20route.%20In%20addition%2C%20the%20MT-associated%20protein%20Mud%5C%2FNuMA%20that%20is%20asymmetrically%20localized%20in%20an%20Asp-dependent%20manner%20at%20the%20nuclear%20envelope%20hemisphere%20where%20MT%20nucleation%20is%20higher%20promotes%20a%20separate%20route.%20Our%20results%20demonstrate%20that%20centrosomes%20do%20not%20provide%20an%20obligatory%20driving%20force%20for%20nuclear%20movement%2C%20but%20together%20with%20Mud%2C%20contribute%20to%20the%20mechanisms%20that%20ensure%20the%20robustness%20of%20asymmetric%20nuclear%20positioning.%22%2C%22date%22%3A%222017-04-27%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1038%5C%2Fncomms15168%22%2C%22ISSN%22%3A%222041-1723%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.nature.com%5C%2Farticles%5C%2Fncomms15168%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222023-06-05T14%3A52%3A21Z%22%7D%7D%2C%7B%22key%22%3A%22TH8ARJNF%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22White%20et%20al.%22%2C%22parsedDate%22%3A%222017-04-01%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BWhite%2C%20P.%20M.%2C%20Serbus%2C%20L.%20R.%2C%20Debec%2C%20A.%2C%20Codina%2C%20A.%2C%20Bray%2C%20W.%2C%20Guichet%2C%20A.%2C%20Lokey%2C%20R.%20S.%2C%20%26amp%3B%20Sullivan%2C%20W.%20%282017%29.%20Reliance%20of%20Wolbachia%20on%20High%20Rates%20of%20Host%20Proteolysis%20Revealed%20by%20a%20Genome-Wide%20RNAi%20Screen%20of%20Drosophila%20Cells.%20%26lt%3Bi%26gt%3BGenetics%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B205%26lt%3B%5C%2Fi%26gt%3B%284%29%2C%201473%26%23×2013%3B1488.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1534%5C%2Fgenetics.116.198903%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1534%5C%2Fgenetics.116.198903%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Reliance%20of%20Wolbachia%20on%20High%20Rates%20of%20Host%20Proteolysis%20Revealed%20by%20a%20Genome-Wide%20RNAi%20Screen%20of%20Drosophila%20Cells%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pamela%20M%22%2C%22lastName%22%3A%22White%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Laura%20R%22%2C%22lastName%22%3A%22Serbus%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Alain%22%2C%22lastName%22%3A%22Debec%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Adan%22%2C%22lastName%22%3A%22Codina%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Walter%22%2C%22lastName%22%3A%22Bray%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22R%20Scott%22%2C%22lastName%22%3A%22Lokey%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22William%22%2C%22lastName%22%3A%22Sullivan%22%7D%5D%2C%22abstractNote%22%3A%22Wolbachia%20are%20gram-negative%2C%20obligate%2C%20intracellular%20bacteria%20carried%20by%20a%20majority%20of%20insect%20species%20worldwide.%20Here%20we%20use%20a%20Wolbachia-infected%20Drosophila%20cell%20line%20and%20genome-wide%20RNA%20interference%20%28RNAi%29%20screening%20to%20identify%20host%20factors%20that%20influence%20Wolbachia%20titer.%20By%20screening%20an%20RNAi%20library%20targeting%2015%2C699%20transcribed%20host%20genes%2C%20we%20identified%2036%20candidate%20genes%20that%20dramatically%20reduced%20Wolbachia%20titer%20and%2041%20that%20increased%20Wolbachia%20titer.%20Host%20gene%20knockdowns%20that%20reduced%20Wolbachia%20titer%20spanned%20a%20broad%20array%20of%20biological%20pathways%20including%20genes%20that%20influenced%20mitochondrial%20function%20and%20lipid%20metabolism.%20In%20addition%2C%20knockdown%20of%20seven%20genes%20in%20the%20host%20ubiquitin%20and%20proteolysis%20pathways%20significantly%20reduced%20Wolbachia%20titer.%20To%20test%20the%20in%20vivo%20relevance%20of%20these%20results%2C%20we%20found%20that%20drug%20and%20mutant%20inhibition%20of%20proteolysis%20reduced%20levels%20of%20Wolbachia%20in%20the%20Drosophila%20oocyte.%20The%20presence%20of%20Wolbachia%20in%20either%20cell%20lines%20or%20oocytes%20dramatically%20alters%20the%20distribution%20and%20abundance%20of%20ubiquitinated%20proteins.%20Functional%20studies%20revealed%20that%20maintenance%20of%20Wolbachia%20titer%20relies%20on%20an%20intact%20host%20Endoplasmic%20Reticulum%20%28ER%29-associated%20protein%20degradation%20pathway%20%28ERAD%29.%20Accordingly%2C%20electron%20microscopy%20studies%20demonstrated%20that%20Wolbachia%20is%20intimately%20associated%20with%20the%20host%20ER%20and%20dramatically%20alters%20the%20morphology%20of%20this%20organelle.%20Given%20Wolbachia%20lack%20essential%20amino%20acid%20biosynthetic%20pathways%2C%20the%20reliance%20of%20Wolbachia%20on%20high%20rates%20of%20host%20proteolysis%20via%20ubiquitination%20and%20the%20ERAD%20pathways%20may%20be%20a%20key%20mechanism%20for%20provisioning%20Wolbachia%20with%20amino%20acids.%20In%20addition%2C%20the%20reliance%20of%20Wolbachia%20on%20the%20ERAD%20pathway%20and%20disruption%20of%20ER%20morphology%20suggests%20a%20previously%20unsuspected%20mechanism%20for%20Wolbachia%5Cu2019s%20potent%20ability%20to%20prevent%20RNA%20virus%20replication.%22%2C%22date%22%3A%222017-04-01%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1534%5C%2Fgenetics.116.198903%22%2C%22ISSN%22%3A%221943-2631%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1534%5C%2Fgenetics.116.198903%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222023-06-05T14%3A52%3A51Z%22%7D%7D%2C%7B%22key%22%3A%22FRZ2Z3ZE%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Jouette%20et%20al.%22%2C%22parsedDate%22%3A%222017%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BJouette%2C%20J.%2C%20Claret%2C%20S.%2C%20%26amp%3B%20Guichet%2C%20A.%20%282017%29.%20Phosphoinositides%20and%20Cell%20Polarity%20in%20the%20Drosophila%20Egg%20Chamber.%20In%20M.%20Kloc%20%28Ed.%29%2C%20%26lt%3Bi%26gt%3BOocytes%3A%20Maternal%20Information%20and%20Functions%26lt%3B%5C%2Fi%26gt%3B%20%28pp.%20169%26%23×2013%3B187%29.%20Springer%20International%20Publishing.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1007%5C%2F978-3-319-60855-6_8%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1007%5C%2F978-3-319-60855-6_8%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22bookSection%22%2C%22title%22%3A%22Phosphoinositides%20and%20Cell%20Polarity%20in%20the%20Drosophila%20Egg%20Chamber%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Julie%22%2C%22lastName%22%3A%22Jouette%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sandra%22%2C%22lastName%22%3A%22Claret%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%2C%7B%22creatorType%22%3A%22editor%22%2C%22firstName%22%3A%22Malgorzata%22%2C%22lastName%22%3A%22Kloc%22%7D%5D%2C%22abstractNote%22%3A%22Phosphatidylinositol%20phosphates%20%28PIPs%29%20are%20essential%20membrane%20components.%20They%20are%20localized%20at%20distinct%20membrane%20domains%20and%20recruit%20distinct%20effectors%3B%20they%20play%20an%20important%20role%20in%20the%20maintenance%20of%20membrane%20identity.%20They%20are%20essential%20for%20many%20cellular%20functions%20that%20include%20membrane%20trafficking%2C%20cytoskeletal%20organization%2C%20cell%20polarity%20and%20tissue%20morphogenesis.%20Cell%20polarity%20is%20also%20controlled%20by%20a%20set%20of%20polarity%20proteins%2C%20the%20PAR%20proteins%2C%20well%20conserved%20among%20bilaterians.%20These%20proteins%20are%20part%20of%20two%20dynamic%20networks%20that%20are%20engaged%20in%20a%20mutual%20negative-feedback%20regulation.%20PAR%20proteins%20control%20cell%20polarity%20by%20regulating%20cytoskeletal%20organization%2C%20asymmetric%20distributions%20of%20cellular%20components%20and%20directional%20transport%20through%20the%20cells.%20They%20share%20common%20activities%20with%20the%20PIPs%20in%20the%20control%20of%20intracellular%20polarity.%20Therefore%2C%20the%20analysis%20of%20potential%20cross%20talks%20between%20polarity%20proteins%20and%20PIPs%20is%20particularly%20important.%20The%20Drosophila%20egg%20chamber%20provides%20a%20very%20good%20model%20system%20to%20study%20the%20processes%20that%20control%20cell%20polarity.%20It%20includes%20the%20oocyte%2C%20a%20large%20cell%20in%20which%20asymmetric%20transport%20is%20very%20easy%20to%20monitor.%20Furthermore%2C%20the%20oocyte%20is%20surrounded%20by%20a%20follicular%20epithelium%20that%20allows%20the%20study%20of%20cross%20talks%20between%20polarity%20and%20tissue%20morphogenesis.%20This%20review%20focuses%20on%20the%20polarization%20of%20Drosophila%20egg%20chamber%20and%20our%20understanding%20of%20PIPs%20requirement%20during%20Drosophila%20oogenesis%20and%20discusses%20the%20relationship%20between%20PIPs%20and%20polarity%20proteins.%22%2C%22bookTitle%22%3A%22Oocytes%3A%20Maternal%20Information%20and%20Functions%22%2C%22date%22%3A%222017%22%2C%22language%22%3A%22en%22%2C%22ISBN%22%3A%22978-3-319-60855-6%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1007%5C%2F978-3-319-60855-6_8%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A07%3A13Z%22%7D%7D%2C%7B%22key%22%3A%22JWDWT9PJ%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Debec%20et%20al.%22%2C%22parsedDate%22%3A%222016%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BDebec%2C%20A.%2C%20Megraw%2C%20T.%20L.%2C%20%26amp%3B%20Guichet%2C%20A.%20%282016%29.%20Methods%20to%20Establish%20Drosophila%20Cell%20Lines.%20In%20C.%20Dahmann%20%28Ed.%29%2C%20%26lt%3Bi%26gt%3BDrosophila%3A%20Methods%20and%20Protocols%26lt%3B%5C%2Fi%26gt%3B%20%28pp.%20333%26%23×2013%3B351%29.%20Springer.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1007%5C%2F978-1-4939-6371-3_21%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1007%5C%2F978-1-4939-6371-3_21%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22bookSection%22%2C%22title%22%3A%22Methods%20to%20Establish%20Drosophila%20Cell%20Lines%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Alain%22%2C%22lastName%22%3A%22Debec%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Timothy%20L.%22%2C%22lastName%22%3A%22Megraw%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%2C%7B%22creatorType%22%3A%22editor%22%2C%22firstName%22%3A%22Christian%22%2C%22lastName%22%3A%22Dahmann%22%7D%5D%2C%22abstractNote%22%3A%22Hundreds%20of%20Drosophila%20cell%20lines%20have%20been%20established%20in%20the%20labs%20of%20many%20researchers%20over%20the%20last%20decades%20and%20have%20been%20important%20tools%20for%20research.%20Although%20these%20cells%20often%20deviate%20from%20normal%20cell%20physiology%20and%20genetic%20composition%2C%20such%20systems%20nonetheless%20are%20powerful%20models%20for%20biochemical%2C%20cell%20biological%2C%20and%20genetics%20studies%20that%20are%20experimentally%20difficult%20in%20vivo.%20While%20published%20descriptions%20of%20cell%20line%20generation%20are%20available%20in%20the%20literature%2C%20how%20to%20generate%20new%20Drosophila%20cell%20lines%20can%20be%20challenging%20for%20beginners.%20Here%2C%20we%20describe%20a%20detailed%2C%20simple%20protocol%20to%20establish%20new%20Drosophila%20cell%20lines.%22%2C%22bookTitle%22%3A%22Drosophila%3A%20Methods%20and%20Protocols%22%2C%22date%22%3A%222016%22%2C%22language%22%3A%22en%22%2C%22ISBN%22%3A%22978-1-4939-6371-3%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1007%5C%2F978-1-4939-6371-3_21%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A08%3A23Z%22%7D%7D%2C%7B%22key%22%3A%229EEDSSRV%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Le%20Droguen%20et%20al.%22%2C%22parsedDate%22%3A%222015-01-15%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BLe%20Droguen%2C%20P.-M.%2C%20Claret%2C%20S.%2C%20Guichet%2C%20A.%2C%20%26amp%3B%20Brodu%2C%20V.%20%282015%29.%20Microtubule-dependent%20apical%20restriction%20of%20recycling%20endosomes%20sustains%20adherens%20junctions%20during%20morphogenesis%20of%20the%20Drosophila%20tracheal%20system.%20%26lt%3Bi%26gt%3BDevelopment%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B142%26lt%3B%5C%2Fi%26gt%3B%282%29%2C%20363%26%23×2013%3B374.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fdev.113472%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fdev.113472%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Microtubule-dependent%20apical%20restriction%20of%20recycling%20endosomes%20sustains%20adherens%20junctions%20during%20morphogenesis%20of%20the%20Drosophila%20tracheal%20system%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pierre-Marie%22%2C%22lastName%22%3A%22Le%20Droguen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sandra%22%2C%22lastName%22%3A%22Claret%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22V%5Cu00e9ronique%22%2C%22lastName%22%3A%22Brodu%22%7D%5D%2C%22abstractNote%22%3A%22Epithelial%20remodelling%20is%20an%20essential%20mechanism%20for%20organogenesis%2C%20during%20which%20cells%20change%20shape%20and%20position%20while%20maintaining%20contact%20with%20each%20other.%20Adherens%20junctions%20%28AJs%29%20mediate%20stable%20intercellular%20cohesion%20but%20must%20be%20actively%20reorganised%20to%20allow%20morphogenesis.%20Vesicle%20trafficking%20and%20the%20microtubule%20%28MT%29%20cytoskeleton%20contribute%20to%20regulating%20AJs%20but%20their%20interrelationship%20remains%20elusive.%20We%20carried%20out%20a%20detailed%20analysis%20of%20the%20role%20of%20MTs%20in%20cell%20remodelling%20during%20formation%20of%20the%20tracheal%20system%20in%20the%20Drosophila%20embryo.%20Induction%20of%20MT%20depolymerisation%20specifically%20in%20tracheal%20cells%20shows%20that%20MTs%20are%20essential%20during%20a%20specific%20time%20frame%20of%20tracheal%20cell%20elongation%20while%20the%20branch%20extends.%20In%20the%20absence%20of%20MTs%2C%20one%20tracheal%20cell%20per%20branch%20overelongates%2C%20ultimately%20leading%20to%20branch%20break.%20Three-dimensional%20quantifications%20revealed%20that%20MTs%20are%20crucial%20to%20sustain%20E-Cadherin%20%28Shotgun%29%20and%20Par-3%20%28Bazooka%29%20levels%20at%20AJs.%20Maintaining%20E-Cadherin%5C%2FPar-3%20levels%20at%20the%20apical%20domain%20requires%20de%20novo%20synthesis%20rather%20than%20internalisation%20and%20recycling%20from%20and%20to%20the%20apical%20plasma%20membrane.%20However%2C%20apical%20targeting%20of%20E-Cadherin%20and%20Par-3%20requires%20functional%20recycling%20endosomes%2C%20suggesting%20an%20intermediate%20role%20for%20this%20compartment%20in%20targeting%20de%20novo%20synthesized%20E-Cadherin%20to%20the%20plasma%20membrane.%20We%20demonstrate%20that%20apical%20enrichment%20of%20recycling%20endosomes%20is%20dependent%20on%20the%20MT%20motor%20Dynein%20and%20essential%20for%20the%20function%20of%20this%20vesicular%20compartment.%20In%20addition%2C%20we%20establish%20that%20E-Cadherin%20dynamics%20and%20MT%20requirement%20differ%20in%20remodelling%20tracheal%20cells%20versus%20planar%20epithelial%20cells.%20Altogether%2C%20our%20results%20uncover%20an%20MT-Dynein-dependent%20apical%20restriction%20of%20recycling%20endosomes%20that%20controls%20adhesion%20by%20sustaining%20Par-3%20and%20E-Cadherin%20levels%20at%20AJs%20during%20morphogenesis.%22%2C%22date%22%3A%222015-01-15%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1242%5C%2Fdev.113472%22%2C%22ISSN%22%3A%220950-1991%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fdev.113472%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A09%3A02Z%22%7D%7D%2C%7B%22key%22%3A%22TDYG4AWJ%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Legent%20et%20al.%22%2C%22parsedDate%22%3A%222015%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BLegent%2C%20K.%2C%20Tissot%2C%20N.%2C%20%26amp%3B%20Guichet%2C%20A.%20%282015%29.%20Visualizing%20Microtubule%20Networks%20During%20Drosophila%20Oogenesis%20Using%20Fixed%20and%20Live%20Imaging.%20In%20D.%20P.%20Bratu%20%26amp%3B%20G.%20P.%20McNeil%20%28Eds.%29%2C%20%26lt%3Bi%26gt%3BDrosophila%20Oogenesis%3A%20Methods%20and%20Protocols%26lt%3B%5C%2Fi%26gt%3B%20%28pp.%2099%26%23×2013%3B112%29.%20Springer.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1007%5C%2F978-1-4939-2851-4_7%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1007%5C%2F978-1-4939-2851-4_7%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22bookSection%22%2C%22title%22%3A%22Visualizing%20Microtubule%20Networks%20During%20Drosophila%20Oogenesis%20Using%20Fixed%20and%20Live%20Imaging%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kevin%22%2C%22lastName%22%3A%22Legent%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nicolas%22%2C%22lastName%22%3A%22Tissot%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%2C%7B%22creatorType%22%3A%22editor%22%2C%22firstName%22%3A%22Diana%20P.%22%2C%22lastName%22%3A%22Bratu%22%7D%2C%7B%22creatorType%22%3A%22editor%22%2C%22firstName%22%3A%22Gerard%20P.%22%2C%22lastName%22%3A%22McNeil%22%7D%5D%2C%22abstractNote%22%3A%22The%20microtubule%20cytoskeleton%20is%20a%20plastic%20network%20of%20polarized%20cables.%20These%20polymers%20of%20tubulin%20provide%20orientated%20routes%20for%20the%20dynamic%20transport%20of%20cytoplasmic%20molecules%20and%20organelles%2C%20through%20which%20cell%20polarity%20is%20established%20and%20maintained.%20The%20role%20of%20microtubule-mediated%20transport%20in%20the%20asymmetric%20localization%20of%20axis%20polarity%20determinants%2C%20in%20the%20Drosophila%20oocyte%2C%20has%20been%20the%20subject%20of%20extensive%20studies%20in%20the%20past%20years.%20However%2C%20imaging%20the%20distribution%20of%20microtubule%20fibers%20in%20a%20large%20cell%2C%20where%20vitellogenesis%20ensures%20the%20uptake%20of%20a%20thick%20and%20hazy%20yolk%2C%20presents%20a%20series%20of%20technical%20challenges.%20This%20chapter%20briefly%20reviews%20some%20of%20these%20aspects%20and%20describes%20two%20methods%20designed%20to%20circumvent%20these%20difficulties.%20We%20provide%20a%20detailed%20protocol%20for%20the%20visualization%20by%20immunohistochemistry%20of%20the%20three-dimensional%20organization%20of%20tubulin%20cables%20in%20the%20oocyte.%20Additionally%2C%20we%20detail%20the%20stepwise%20procedure%20for%20the%20live%20imaging%20of%20microtubule%20dynamics%20and%20network%20remodeling%2C%20using%20fluorescently%20labeled%20microtubule-associated%20proteins.%22%2C%22bookTitle%22%3A%22Drosophila%20Oogenesis%3A%20Methods%20and%20Protocols%22%2C%22date%22%3A%222015%22%2C%22language%22%3A%22en%22%2C%22ISBN%22%3A%22978-1-4939-2851-4%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1007%5C%2F978-1-4939-2851-4_7%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A08%3A42Z%22%7D%7D%2C%7B%22key%22%3A%22TIB6YG29%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Claret%20et%20al.%22%2C%22parsedDate%22%3A%222014-05-19%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BClaret%2C%20S.%2C%20Jouette%2C%20J.%2C%20Benoit%2C%20B.%2C%20Legent%2C%20K.%2C%20%26amp%3B%20Guichet%2C%20A.%20%282014%29.%20PI%284%2C5%29P2%20Produced%20by%20the%20PI4P5K%20SKTL%20Controls%20Apical%20Size%20by%20Tethering%20PAR-3%20in%20%26lt%3Bi%26gt%3BDrosophila%26lt%3B%5C%2Fi%26gt%3B%20Epithelial%20Cells.%20%26lt%3Bi%26gt%3BCurrent%20Biology%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B24%26lt%3B%5C%2Fi%26gt%3B%2810%29%2C%201071%26%23×2013%3B1079.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.cub.2014.03.056%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.cub.2014.03.056%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22PI%284%2C5%29P2%20Produced%20by%20the%20PI4P5K%20SKTL%20Controls%20Apical%20Size%20by%20Tethering%20PAR-3%20in%20%3Ci%3EDrosophila%3C%5C%2Fi%3E%20Epithelial%20Cells%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sandra%22%2C%22lastName%22%3A%22Claret%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Julie%22%2C%22lastName%22%3A%22Jouette%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22B%5Cu00e9atrice%22%2C%22lastName%22%3A%22Benoit%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kevin%22%2C%22lastName%22%3A%22Legent%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%5D%2C%22abstractNote%22%3A%22Background%5CnThe%20control%20of%20apical-basal%20polarity%20in%20epithelial%20layers%20is%20a%20fundamental%20event%20in%20many%20processes%2C%20ranging%20from%20embryonic%20development%20to%20tumor%20formation.%20A%20key%20feature%20of%20polarized%20epithelial%20cells%20is%20their%20ability%20to%20maintain%20an%20asymmetric%20distribution%20of%20specific%20molecular%20complexes%2C%20including%20the%20phosphoinositides%20PI%284%2C5%29P2%20and%20PI%283%2C4%2C5%29P3.%20The%20spatiotemporal%20regulation%20of%20these%20phosphoinositides%20is%5Cu00a0controlled%20by%20the%20concerted%20action%20of%20phosphoinositide%20kinases%20and%20phosphatases.%5CnResults%5CnUsing%20the%20Drosophila%20follicular%20epithelium%20as%20a%20model%20system%20in%5Cu00a0vivo%2C%20we%20show%20here%20that%20PI%284%2C5%29P2%20is%20crucial%20to%20maintain%20apical-basal%20polarity.%20PI%284%2C5%29P2%20is%20essentially%20regulated%20by%5Cu00a0the%20PI4P5%20kinase%20Skittles%20%28SKTL%29%2C%20whereas%20neither%20the%20phosphatase%20PTEN%20nor%20the%20PI%284%2C5%29P3%20kinase%20DP110%20lead%20to%20loss%20of%20apical-basal%20polarity.%20By%20inactivating%20SKTL%20and%20thereby%20strongly%20reducing%20PI%284%2C5%29P2%20levels%20in%20a%20single%20cell%20of%20the%20epithelium%2C%20we%20observe%20the%20disassembly%20of%20adherens%20junctions%2C%20actin%20cytoskeleton%20reorganization%2C%20and%20apical%20constriction%20leading%20to%20delamination%2C%20a%20process%20similar%20to%20that%20observed%20during%20epithelial-mesenchymal%20transition.%20We%20provide%20evidence%20that%20PI%284%2C5%29P2%20controls%20the%20apical%20targeting%20of%20PAR-3%5C%2FBazooka%20to%20the%20plasma%20membrane%20and%20that%20the%20loss%20of%20this%20polarized%20distribution%20is%20sufficient%20to%20induce%20a%20similar%20cell%20shape%20change.%20Finally%2C%20we%20show%20that%20PI%284%2C5%29P2%20is%20excluded%20from%20the%20cell%20apex%20and%20that%20PAR-3%20diffuses%20laterally%20just%20prior%20to%20the%20apical%20constriction%20in%20a%5Cu00a0context%20of%20endogenous%20invagination.%5CnConclusions%5CnAll%20together%2C%20these%20results%20indicate%20that%20the%20PIP5%20kinase%20SKTL%2C%20by%20controlling%20PI%284%2C5%29P2%20polarity%2C%20regulates%20PAR-3%20localization%20and%20thus%20the%20size%20of%20the%20apical%20domain.%22%2C%22date%22%3A%222014-05-19%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.cub.2014.03.056%22%2C%22ISSN%22%3A%220960-9822%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.sciencedirect.com%5C%2Fscience%5C%2Farticle%5C%2Fpii%5C%2FS0960982214003480%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A25%3A37Z%22%7D%7D%2C%7B%22key%22%3A%22QY7GF5XX%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Lecland%20et%20al.%22%2C%22parsedDate%22%3A%222013-01-17%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BLecland%2C%20N.%2C%20Debec%2C%20A.%2C%20Delmas%2C%20A.%2C%20Moutinho-Pereira%2C%20S.%2C%20Malmanche%2C%20N.%2C%20Bouissou%2C%20A.%2C%20Dupr%26%23xE9%3B%2C%20C.%2C%20Jourdan%2C%20A.%2C%20Raynaud-Messina%2C%20B.%2C%20Maiato%2C%20H.%2C%20%26amp%3B%20Guichet%2C%20A.%20%282013%29.%20Establishment%20and%20mitotic%20characterization%20of%20new%20Drosophila%20acentriolar%20cell%20lines%20from%20DSas-4%20mutant.%20%26lt%3Bi%26gt%3BBiology%20Open%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B2%26lt%3B%5C%2Fi%26gt%3B%283%29%2C%20314%26%23×2013%3B323.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fbio.20133327%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fbio.20133327%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Establishment%20and%20mitotic%20characterization%20of%20new%20Drosophila%20acentriolar%20cell%20lines%20from%20DSas-4%20mutant%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nicolas%22%2C%22lastName%22%3A%22Lecland%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Alain%22%2C%22lastName%22%3A%22Debec%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Audrey%22%2C%22lastName%22%3A%22Delmas%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sara%22%2C%22lastName%22%3A%22Moutinho-Pereira%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nicolas%22%2C%22lastName%22%3A%22Malmanche%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anais%22%2C%22lastName%22%3A%22Bouissou%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Cl%5Cu00e9mence%22%2C%22lastName%22%3A%22Dupr%5Cu00e9%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Aimie%22%2C%22lastName%22%3A%22Jourdan%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Brigitte%22%2C%22lastName%22%3A%22Raynaud-Messina%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Helder%22%2C%22lastName%22%3A%22Maiato%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%5D%2C%22abstractNote%22%3A%22In%20animal%20cells%20the%20centrosome%20is%20commonly%20viewed%20as%20the%20main%20cellular%20structure%20driving%20microtubule%20%28MT%29%20assembly%20into%20the%20mitotic%20spindle%20apparatus.%20However%2C%20additional%20pathways%2C%20such%20as%20those%20mediated%20by%20chromatin%20and%20augmin%2C%20are%20involved%20in%20the%20establishment%20of%20functional%20spindles.%20The%20molecular%20mechanisms%20involved%20in%20these%20pathways%20remain%20poorly%20understood%2C%20mostly%20due%20to%20limitations%20inherent%20to%20current%20experimental%20systems%20available.%20To%20overcome%20these%20limitations%20we%20have%20developed%20six%20new%20Drosophila%20cell%20lines%20derived%20from%20Drosophila%20homozygous%20mutants%20for%20DSas-4%2C%20a%20protein%20essential%20for%20centriole%20biogenesis.%20These%20cells%20lack%20detectable%20centrosomal%20structures%2C%20astral%20MT%2C%20with%20dispersed%20pericentriolar%20proteins%20D-PLP%2C%20Centrosomin%20and%20%5Cu03b3-tubulin.%20They%20show%20poorly%20focused%20spindle%20poles%20that%20reach%20the%20plasma%20membrane.%20Despite%20being%20compromised%20for%20functional%20centrosome%2C%20these%20cells%20could%20successfully%20undergo%20mitosis.Live-cell%20imaging%20analysis%20of%20acentriolar%20spindle%20assembly%20revealed%20that%20nascent%20MTs%20are%20nucleated%20from%20multiple%20points%20in%20the%20vicinity%20of%20chromosomes.%20These%20nascent%20MTs%20then%20grow%20away%20from%20kinetochores%20allowing%20the%20expansion%20of%20fibers%20that%20will%20be%20part%20of%20the%20future%20acentriolar%20spindle.%20MT%20repolymerization%20assays%20illustrate%20that%20acentriolar%20spindle%20assembly%20occurs%20%5Cu201cinside-out%5Cu201d%20from%20the%20chromosomes.%20Colchicine-mediated%20depolymerization%20of%20MTs%20further%20revealed%20the%20presence%20of%20a%20functional%20Spindle%20Assembly%20Checkpoint%20%28SAC%29%20in%20the%20acentriolar%20cells.%20Finally%2C%20pilot%20RNAi%20experiments%20open%20the%20potential%20use%20of%20these%20cell%20lines%20for%20the%20molecular%20dissection%20of%20anastral%20pathways%20in%20spindle%20and%20centrosome%20assembly.%22%2C%22date%22%3A%222013-01-17%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1242%5C%2Fbio.20133327%22%2C%22ISSN%22%3A%222046-6390%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fbio.20133327%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A23%3A15Z%22%7D%7D%2C%7B%22key%22%3A%22V4VQRZFS%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Baffet%20et%20al.%22%2C%22parsedDate%22%3A%222012-09-15%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BBaffet%2C%20A.%20D.%2C%20Benoit%2C%20B.%2C%20Januschke%2C%20J.%2C%20Audo%2C%20J.%2C%20Gourhand%2C%20V.%2C%20Roth%2C%20S.%2C%20%26amp%3B%20Guichet%2C%20A.%20%282012%29.%20Drosophila%20tubulin-binding%20cofactor%20B%20is%20required%20for%20microtubule%20network%20formation%20and%20for%20cell%20polarity.%20%26lt%3Bi%26gt%3BMolecular%20Biology%20of%20the%20Cell%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B23%26lt%3B%5C%2Fi%26gt%3B%2818%29%2C%203591%26%23×2013%3B3601.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1091%5C%2Fmbc.e11-07-0633%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1091%5C%2Fmbc.e11-07-0633%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Drosophila%20tubulin-binding%20cofactor%20B%20is%20required%20for%20microtubule%20network%20formation%20and%20for%20cell%20polarity%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Alexandre%20D.%22%2C%22lastName%22%3A%22Baffet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22B%5Cu00e9atrice%22%2C%22lastName%22%3A%22Benoit%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jens%22%2C%22lastName%22%3A%22Januschke%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jennifer%22%2C%22lastName%22%3A%22Audo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Vanessa%22%2C%22lastName%22%3A%22Gourhand%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Siegfried%22%2C%22lastName%22%3A%22Roth%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%5D%2C%22abstractNote%22%3A%22Microtubules%20%28MTs%29%20are%20essential%20for%20cell%20division%2C%20shape%2C%20intracellular%20transport%2C%20and%20polarity.%20MT%20stability%20is%20regulated%20by%20many%20factors%2C%20including%20MT-associated%20proteins%20and%20proteins%20controlling%20the%20amount%20of%20free%20tubulin%20heterodimers%20available%20for%20polymerization.%20Tubulin-binding%20cofactors%20are%20potential%20key%20regulators%20of%20free%20tubulin%20concentration%2C%20since%20they%20are%20required%20for%20%5Cu03b1-%5Cu03b2%5Cu2013tubulin%20dimerization%20in%20vitro.%20In%20this%20paper%2C%20we%20show%20that%20mutation%20of%20the%20Drosophila%20tubulin-binding%20cofactor%20B%20%28dTBCB%29%20affects%20the%20levels%20of%20both%20%5Cu03b1-%20and%20%5Cu03b2-tubulins%20and%20dramatically%20destabilizes%20the%20MT%20network%20in%20different%20fly%20tissues.%20However%2C%20we%20find%20that%20dTBCB%20is%20dispensable%20for%20the%20early%20MT-dependent%20steps%20of%20oogenesis%2C%20including%20cell%20division%2C%20and%20that%20dTBCB%20is%20not%20required%20for%20mitosis%20in%20several%20tissues.%20In%20striking%20contrast%2C%20the%20absence%20of%20dTBCB%20during%20later%20stages%20of%20oogenesis%20causes%20major%20defects%20in%20cell%20polarity.%20We%20show%20that%20dTBCB%20is%20required%20for%20the%20polarized%20localization%20of%20the%20axis-determining%20mRNAs%20within%20the%20oocyte%20and%20for%20the%20apico-basal%20polarity%20of%20the%20surrounding%20follicle%20cells.%20These%20results%20establish%20a%20developmental%20function%20for%20the%20dTBCB%20gene%20that%20is%20essential%20for%20viability%20and%20MT-dependent%20cell%20polarity%2C%20but%20not%20cell%20division.%22%2C%22date%22%3A%222012-09-15%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1091%5C%2Fmbc.e11-07-0633%22%2C%22ISSN%22%3A%221059-1524%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.molbiolcell.org%5C%2Fdoi%5C%2F10.1091%5C%2Fmbc.e11-07-0633%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A10%3A09Z%22%7D%7D%2C%7B%22key%22%3A%22YBMU7999%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Parrott%20et%20al.%22%2C%22parsedDate%22%3A%222011-09-16%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BParrott%2C%20B.%20B.%2C%20Chiang%2C%20Y.%2C%20Hudson%2C%20A.%2C%20Sarkar%2C%20A.%2C%20Guichet%2C%20A.%2C%20%26amp%3B%20Schulz%2C%20C.%20%282011%29.%20Nucleoporin98-96%20Function%20Is%20Required%20for%20Transit%20Amplification%20Divisions%20in%20the%20Germ%20Line%20of%20Drosophila%20melanogaster.%20%26lt%3Bi%26gt%3BPLOS%20ONE%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B6%26lt%3B%5C%2Fi%26gt%3B%289%29%2C%20e25087.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1371%5C%2Fjournal.pone.0025087%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1371%5C%2Fjournal.pone.0025087%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Nucleoporin98-96%20Function%20Is%20Required%20for%20Transit%20Amplification%20Divisions%20in%20the%20Germ%20Line%20of%20Drosophila%20melanogaster%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Benjamin%20B.%22%2C%22lastName%22%3A%22Parrott%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yuting%22%2C%22lastName%22%3A%22Chiang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Alicia%22%2C%22lastName%22%3A%22Hudson%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Angshuman%22%2C%22lastName%22%3A%22Sarkar%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Cordula%22%2C%22lastName%22%3A%22Schulz%22%7D%5D%2C%22abstractNote%22%3A%22Production%20of%20specialized%20cells%20from%20precursors%20depends%20on%20a%20tightly%20regulated%20sequence%20of%20proliferation%20and%20differentiation%20steps.%20In%20the%20gonad%20of%20Drosophila%20melanogaster%2C%20the%20daughters%20of%20germ%20line%20stem%20cells%20%28GSC%29%20go%20through%20precisely%20four%20rounds%20of%20transit%20amplification%20divisions%20to%20produce%20clusters%20of%2016%20interconnected%20germ%20line%20cells%20before%20entering%20a%20stereotypic%20differentiation%20cascade.%20Here%20we%20show%20that%20animals%20harbouring%20a%20transposon%20insertion%20in%20the%20center%20of%20the%20complex%20nucleoporin98-96%20%28nup98-96%29%20locus%20had%20severe%20defects%20in%20the%20early%20steps%20of%20this%20developmental%20program%2C%20ultimately%20leading%20to%20germ%20cell%20loss%20and%20sterility.%20A%20phenotypic%20analysis%20indicated%20that%20flies%20carrying%20the%20transposon%20insertion%2C%20designated%20nup98-962288%2C%20had%20dramatically%20reduced%20numbers%20of%20germ%20line%20cells.%20In%20contrast%20to%20controls%2C%20mutant%20testes%20contained%20many%20solitary%20germ%20line%20cells%20that%20had%20committed%20to%20differentiation%20as%20well%20as%20abnormally%20small%20clusters%20of%20two%2C%20four%20or%20eight%20differentiating%20germ%20line%20cells.%20This%20indicates%20that%20mutant%20GSCs%20rather%20differentiated%20than%20self-renewed%2C%20and%20that%20these%20GSCs%20and%20their%20daughters%20initiated%20the%20differentiation%20cascade%20after%20zero%2C%20or%20less%20than%20four%20rounds%20of%20amplification%20divisions.%20This%20phenotype%20remained%20unaffected%20by%20hyper-activation%20of%20signalling%20pathways%20that%20normally%20result%20in%20excessive%20proliferation%20of%20GSCs%20and%20their%20daughters.%20Expression%20of%20wildtype%20nup98-96%20specifically%20in%20the%20germ%20line%20cells%20of%20mutant%20animals%20fully%20restored%20development%20of%20the%20GSC%20lineage%2C%20demonstrating%20that%20the%20effect%20of%20the%20mutation%20is%20cell-autonomous.%20Nucleoporins%20are%20the%20structural%20components%20of%20the%20nucleopore%20and%20have%20also%20been%20implicated%20in%20transcriptional%20regulation%20of%20specific%20target%20genes.%20The%20nuclear%20envelopes%20of%20germ%20cells%20and%20general%20nucleocytoplasmic%20transport%20in%20nup98-96%20mutant%20animals%20appeared%20normal%2C%20leading%20us%20to%20propose%20that%20Drosophila%20nup98-96%20mediates%20the%20transport%20or%20transcription%20of%20targets%20required%20for%20the%20developmental%20timing%20between%20amplification%20and%20differentiation.%22%2C%22date%22%3A%2216%20sept.%202011%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1371%5C%2Fjournal.pone.0025087%22%2C%22ISSN%22%3A%221932-6203%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fjournals.plos.org%5C%2Fplosone%5C%2Farticle%3Fid%3D10.1371%5C%2Fjournal.pone.0025087%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A24%3A09Z%22%7D%7D%2C%7B%22key%22%3A%22CHIV293K%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Brodu%20et%20al.%22%2C%22parsedDate%22%3A%222010-05-18%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BBrodu%2C%20V.%2C%20Baffet%2C%20A.%20D.%2C%20Le%20Droguen%2C%20P.-M.%2C%20Casanova%2C%20J.%2C%20%26amp%3B%20Guichet%2C%20A.%20%282010%29.%20A%20Developmentally%20Regulated%20Two-Step%20Process%20Generates%20a%20Noncentrosomal%20Microtubule%20Network%20in%20%26lt%3Bi%26gt%3BDrosophila%26lt%3B%5C%2Fi%26gt%3B%20Tracheal%20Cells.%20%26lt%3Bi%26gt%3BDevelopmental%20Cell%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B18%26lt%3B%5C%2Fi%26gt%3B%285%29%2C%20790%26%23×2013%3B801.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.devcel.2010.03.015%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.devcel.2010.03.015%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22A%20Developmentally%20Regulated%20Two-Step%20Process%20Generates%20a%20Noncentrosomal%20Microtubule%20Network%20in%20%3Ci%3EDrosophila%3C%5C%2Fi%3E%20Tracheal%20Cells%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22V%5Cu00e9ronique%22%2C%22lastName%22%3A%22Brodu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Alexandre%20D.%22%2C%22lastName%22%3A%22Baffet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pierre-Marie%22%2C%22lastName%22%3A%22Le%20Droguen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jordi%22%2C%22lastName%22%3A%22Casanova%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%5D%2C%22abstractNote%22%3A%22Microtubules%20%28MTs%29%20are%20essential%20for%20many%20cell%20features%2C%20such%20as%20polarity%2C%20motility%2C%20shape%2C%20and%20vesicle%20trafficking.%20Therefore%2C%20in%20a%20multicellular%20organism%2C%20their%20organization%20differs%20between%20cell%20types%20and%20during%20development%3B%20however%2C%20the%20control%20of%20this%20process%20remains%20elusive.%20Here%2C%20we%20show%20that%20during%20Drosophila%20tracheal%20morphogenesis%2C%20MT%20reorganization%20is%20coupled%20to%20relocalization%20of%20the%20microtubule%20organizing%20centers%20%28MTOC%29%20components%20from%20the%20centrosome%20to%20the%20apical%20cell%20domain%20from%20where%20MTs%20then%20grow.%20We%20reveal%20that%20this%20process%20is%20controlled%20by%20the%20trachealess%20patterning%20gene%20in%20a%20two-step%20mechanism.%20MTOC%20components%20are%20first%20released%20from%20the%20centrosome%20by%20the%20activity%20of%20the%5Cu00a0MT-severing%20protein%20Spastin%2C%20and%20then%20anchored%20apically%20through%20the%20transmembrane%20protein%20Piopio.%20We%20further%20show%20that%20these%20changes%20are%20essential%20for%20tracheal%20development%2C%20thus%20stressing%20the%20functional%20relevance%20of%20MT%20reorganization%20for%20morphogenesis.%22%2C%22date%22%3A%222010-05-18%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.devcel.2010.03.015%22%2C%22ISSN%22%3A%221534-5807%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.sciencedirect.com%5C%2Fscience%5C%2Farticle%5C%2Fpii%5C%2FS1534580710001590%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A21%3A56Z%22%7D%7D%2C%7B%22key%22%3A%22DB2ASMUQ%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Fabian%20et%20al.%22%2C%22parsedDate%22%3A%222010-05%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BFabian%2C%20L.%2C%20Wei%2C%20H.-C.%2C%20Rollins%2C%20J.%2C%20Noguchi%2C%20T.%2C%20Blankenship%2C%20J.%20T.%2C%20Bellamkonda%2C%20K.%2C%20Polevoy%2C%20G.%2C%20Gervais%2C%20L.%2C%20Guichet%2C%20A.%2C%20Fuller%2C%20M.%20T.%2C%20%26amp%3B%20Brill%2C%20J.%20A.%20%282010%29.%20Phosphatidylinositol%204%2C5-bisphosphate%20Directs%20Spermatid%20Cell%20Polarity%20and%20Exocyst%20Localization%20in%20Drosophila.%20%26lt%3Bi%26gt%3BMolecular%20Biology%20of%20the%20Cell%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B21%26lt%3B%5C%2Fi%26gt%3B%289%29%2C%201546%26%23×2013%3B1555.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1091%5C%2Fmbc.e09-07-0582%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1091%5C%2Fmbc.e09-07-0582%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Phosphatidylinositol%204%2C5-bisphosphate%20Directs%20Spermatid%20Cell%20Polarity%20and%20Exocyst%20Localization%20in%20Drosophila%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Lacramioara%22%2C%22lastName%22%3A%22Fabian%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ho-Chun%22%2C%22lastName%22%3A%22Wei%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Janet%22%2C%22lastName%22%3A%22Rollins%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Tatsuhiko%22%2C%22lastName%22%3A%22Noguchi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J.%20Todd%22%2C%22lastName%22%3A%22Blankenship%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kishan%22%2C%22lastName%22%3A%22Bellamkonda%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Gordon%22%2C%22lastName%22%3A%22Polevoy%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Louis%22%2C%22lastName%22%3A%22Gervais%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Margaret%20T.%22%2C%22lastName%22%3A%22Fuller%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Julie%20A.%22%2C%22lastName%22%3A%22Brill%22%7D%5D%2C%22abstractNote%22%3A%22During%20spermiogenesis%2C%20Drosophila%20melanogaster%20spermatids%20coordinate%20their%20elongation%20in%20interconnected%20cysts%20that%20become%20highly%20polarized%2C%20with%20nuclei%20localizing%20to%20one%20end%20and%20sperm%20tail%20growth%20occurring%20at%20the%20other.%20Remarkably%20little%20is%20known%20about%20the%20signals%20that%20drive%20spermatid%20polarity%20and%20elongation.%20Here%20we%20identify%20phosphoinositides%20as%20critical%20regulators%20of%20these%20processes.%20Reduction%20of%20plasma%20membrane%20phosphatidylinositol%204%2C5-bisphosphate%20%28PIP2%29%20by%20low-level%20expression%20of%20the%20PIP2%20phosphatase%20SigD%20or%20mutation%20of%20the%20PIP2%20biosynthetic%20enzyme%20Skittles%20%28Sktl%29%20results%20in%20dramatic%20defects%20in%20spermatid%20cysts%2C%20which%20become%20bipolar%20and%20fail%20to%20fully%20elongate.%20Defects%20in%20polarity%20are%20evident%20from%20the%20earliest%20stages%20of%20elongation%2C%20indicating%20that%20phosphoinositides%20are%20required%20for%20establishment%20of%20polarity.%20Sktl%20and%20PIP2%20localize%20to%20the%20growing%20end%20of%20the%20cysts%20together%20with%20the%20exocyst%20complex.%20Strikingly%2C%20the%20exocyst%20becomes%20completely%20delocalized%20when%20PIP2%20levels%20are%20reduced%2C%20and%20overexpression%20of%20Sktl%20restores%20exocyst%20localization%20and%20spermatid%20cyst%20polarity.%20Moreover%2C%20the%20exocyst%20is%20required%20for%20polarity%2C%20as%20partial%20loss%20of%20function%20of%20the%20exocyst%20subunit%20Sec8%20results%20in%20bipolar%20cysts.%20Our%20data%20are%20consistent%20with%20a%20mechanism%20in%20which%20localized%20synthesis%20of%20PIP2%20recruits%20the%20exocyst%20to%20promote%20targeted%20membrane%20delivery%20and%20polarization%20of%20the%20elongating%20cysts.%22%2C%22date%22%3A%222010-05%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1091%5C%2Fmbc.e09-07-0582%22%2C%22ISSN%22%3A%221059-1524%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.molbiolcell.org%5C%2Fdoi%5C%2F10.1091%5C%2Fmbc.e09-07-0582%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A12%3A57Z%22%7D%7D%2C%7B%22key%22%3A%22YAIS7WGQ%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Lachkar%20et%20al.%22%2C%22parsedDate%22%3A%222010-04-09%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BLachkar%2C%20S.%2C%20Lebois%2C%20M.%2C%20Steinmetz%2C%20M.%20O.%2C%20Guichet%2C%20A.%2C%20Lal%2C%20N.%2C%20Curmi%2C%20P.%20A.%2C%20Sobel%2C%20A.%2C%20%26amp%3B%20Ozon%2C%20S.%20%282010%29.%20Drosophila%20Stathmins%20Bind%20Tubulin%20Heterodimers%20with%20High%20and%20Variable%20Stoichiometries.%20%26lt%3Bi%26gt%3BThe%20Journal%20of%20Biological%20Chemistry%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B285%26lt%3B%5C%2Fi%26gt%3B%2815%29%2C%2011667%26%23×2013%3B11680.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1074%5C%2Fjbc.M109.096727%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1074%5C%2Fjbc.M109.096727%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Drosophila%20Stathmins%20Bind%20Tubulin%20Heterodimers%20with%20High%20and%20Variable%20Stoichiometries%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sylvie%22%2C%22lastName%22%3A%22Lachkar%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Marion%22%2C%22lastName%22%3A%22Lebois%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Michel%20O.%22%2C%22lastName%22%3A%22Steinmetz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Neha%22%2C%22lastName%22%3A%22Lal%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Patrick%20A.%22%2C%22lastName%22%3A%22Curmi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Andr%5Cu00e9%22%2C%22lastName%22%3A%22Sobel%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sylvie%22%2C%22lastName%22%3A%22Ozon%22%7D%5D%2C%22abstractNote%22%3A%22In%20vertebrates%2C%20stathmins%20form%20a%20family%20of%20proteins%20possessing%20two%20tubulin%20binding%20repeats%20%28TBRs%29%2C%20which%20each%20binds%20one%20soluble%20tubulin%20heterodimer.%20The%20stathmins%20thus%20sequester%20two%20tubulins%20in%20a%20phosphorylation-dependent%20manner%2C%20providing%20a%20link%20between%20signal%20transduction%20and%20microtubule%20dynamics.%20In%20Drosophila%2C%20we%20show%20here%20that%20a%20single%20stathmin%20gene%20%28stai%29%20encodes%20a%20family%20of%20D-stathmin%20proteins.%20Two%20of%20the%20D-stathmins%20are%20maternally%20deposited%20and%20then%20restricted%20to%20germ%20cells%2C%20and%20the%20other%20two%20are%20detected%20in%20the%20nervous%20system%20during%20embryo%20development.%20Like%20in%20vertebrates%2C%20the%20nervous%20system-enriched%20stathmins%20contain%20an%20N-terminal%20domain%20involved%20in%20subcellular%20targeting.%20All%20the%20D-stathmins%20possess%20a%20domain%20containing%20three%20or%20four%20predicted%20TBRs%2C%20and%20we%20demonstrate%20here%2C%20using%20complementary%20biochemical%20and%20biophysical%20methods%2C%20that%20all%20four%20predicted%20TBR%20domains%20actually%20bind%20tubulin.%20D-stathmins%20can%20indeed%20bind%20up%20to%20four%20tubulins%2C%20the%20resulting%20complex%20being%20directly%20visualized%20by%20electron%20microscopy.%20Phylogenetic%20analysis%20shows%20that%20the%20presence%20of%20regulated%20multiple%20tubulin%20sites%20is%20a%20conserved%20characteristic%20of%20stathmins%20in%20invertebrates%20and%20allows%20us%20to%20predict%20key%20residues%20in%20stathmin%20for%20the%20binding%20of%20tubulin.%20Altogether%2C%20our%20results%20reveal%20that%20the%20single%20Drosophila%20stathmin%20gene%20codes%20for%20a%20stathmin%20family%20similar%20to%20the%20multigene%20vertebrate%20one%2C%20but%20with%20particular%20tubulin%20binding%20properties.%22%2C%22date%22%3A%222010-4-9%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1074%5C%2Fjbc.M109.096727%22%2C%22ISSN%22%3A%220021-9258%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.ncbi.nlm.nih.gov%5C%2Fpmc%5C%2Farticles%5C%2FPMC2857042%5C%2F%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A13%3A20Z%22%7D%7D%2C%7B%22key%22%3A%22U9YX8ZCT%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Compagnon%20et%20al.%22%2C%22parsedDate%22%3A%222009-01-01%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BCompagnon%2C%20J.%2C%20Gervais%2C%20L.%2C%20Roman%2C%20M.%20S.%2C%20Chamot-B%26%23×153%3Buf%2C%20S.%2C%20%26amp%3B%20Guichet%2C%20A.%20%282009%29.%20Interplay%20between%20Rab5%20and%20PtdIns%284%2C5%29P2%20controls%20early%20endocytosis%20in%20the%20Drosophila%20germline.%20%26lt%3Bi%26gt%3BJournal%20of%20Cell%20Science%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B122%26lt%3B%5C%2Fi%26gt%3B%281%29%2C%2025%26%23×2013%3B35.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fjcs.033027%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fjcs.033027%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Interplay%20between%20Rab5%20and%20PtdIns%284%2C5%29P2%20controls%20early%20endocytosis%20in%20the%20Drosophila%20germline%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Julien%22%2C%22lastName%22%3A%22Compagnon%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Louis%22%2C%22lastName%22%3A%22Gervais%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mabel%20San%22%2C%22lastName%22%3A%22Roman%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sophy%22%2C%22lastName%22%3A%22Chamot-B%5Cu0153uf%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%5D%2C%22abstractNote%22%3A%22Phosphoinositides%20have%20emerged%20as%20key%20regulators%20of%20membrane%20traffic%20through%20their%20control%20of%20the%20localization%20and%20activity%20of%20several%20effector%20proteins.%20Both%20Rab5%20and%20phosphatidylinositol%20%284%2C5%29-bisphosphate%20%5BPtdIns%284%2C5%29P2%5D%20are%20involved%20in%20the%20early%20steps%20of%20the%20clathrin-dependent%20endocytic%20pathway%2C%20but%20little%20is%20known%20about%20how%20their%20functions%20are%20coordinated.%20We%20have%20studied%20the%20role%20of%20PtdIns%284%2C5%29P2%20and%20Rab5%20in%20the%20Drosophila%20germline%20during%20oogenesis.%20We%20found%20that%20Rab5%20is%20required%20for%20the%20maturation%20of%20early%20endocytic%20vesicles.%20We%20show%20that%20PtdIns%284%2C5%29P2%20is%20required%20for%20endocytic-vesicle%20formation%2C%20for%20Rab5%20recruitment%20to%20endosomes%20and%2C%20consistently%2C%20for%20endocytosis.%20Furthermore%2C%20we%20reveal%20a%20previously%20undescribed%20role%20of%20Rab5%20in%20releasing%20PtdIns%284%2C5%29P2%2C%20PtdIns%284%2C5%29P2-binding%20budding%20factors%20and%20F-actin%20from%20early%20endocytic%20vesicles.%20Finally%2C%20we%20show%20that%20overexpressing%20the%20PtdIns%284%2C5%29P2-synthesizing%20enzyme%20Skittles%20leads%20to%20an%20endocytic%20defect%20that%20is%20similar%20to%20that%20seen%20in%20rab5%20loss-of-function%20mutants.%20Hence%2C%20our%20results%20argue%20strongly%20in%20favor%20of%20the%20hypothesis%20that%20the%20Rab5-dependant%20release%20of%20PtdIns%284%2C5%29P2%20from%20endosomes%20that%20we%20discovered%20in%20this%20study%20is%20crucial%20for%20endocytosis%20to%20proceed.%22%2C%22date%22%3A%222009-01-01%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1242%5C%2Fjcs.033027%22%2C%22ISSN%22%3A%220021-9533%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fjcs.033027%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A13%3A37Z%22%7D%7D%2C%7B%22key%22%3A%2272Y4RDEL%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Nicolas%20et%20al.%22%2C%22parsedDate%22%3A%222009-01%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BNicolas%2C%20E.%2C%20Chenouard%2C%20N.%2C%20Olivo-Marin%2C%20J.-C.%2C%20%26amp%3B%20Guichet%2C%20A.%20%282009%29.%20A%20Dual%20Role%20for%20Actin%20and%20Microtubule%20Cytoskeleton%20in%20the%20Transport%20of%20Golgi%20Units%20from%20the%20Nurse%20Cells%20to%20the%20Oocyte%20Across%20Ring%20Canals.%20%26lt%3Bi%26gt%3BMolecular%20Biology%20of%20the%20Cell%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B20%26lt%3B%5C%2Fi%26gt%3B%281%29%2C%20556%26%23×2013%3B568.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1091%5C%2Fmbc.e08-04-0360%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1091%5C%2Fmbc.e08-04-0360%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22A%20Dual%20Role%20for%20Actin%20and%20Microtubule%20Cytoskeleton%20in%20the%20Transport%20of%20Golgi%20Units%20from%20the%20Nurse%20Cells%20to%20the%20Oocyte%20Across%20Ring%20Canals%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Emmanuelle%22%2C%22lastName%22%3A%22Nicolas%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nicolas%22%2C%22lastName%22%3A%22Chenouard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jean-Christophe%22%2C%22lastName%22%3A%22Olivo-Marin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%5D%2C%22abstractNote%22%3A%22Axis%20specification%20during%20Drosophila%20embryonic%20development%20requires%20transfer%20of%20maternal%20components%20during%20oogenesis%20from%20nurse%20cells%20%28NCs%29%20into%20the%20oocyte%20through%20cytoplasmic%20bridges.%20We%20found%20that%20the%20asymmetrical%20distribution%20of%20Golgi%2C%20between%20nurse%20cells%20and%20the%20oocyte%2C%20is%20sustained%20by%20an%20active%20transport%20process.%20We%20have%20characterized%20actin%20basket%20structures%20that%20asymmetrically%20cap%20the%20NC%20side%20of%20Ring%20canals%20%28RCs%29%20connecting%20the%20oocyte.%20Our%20results%20suggest%20that%20these%20actin%20baskets%20structurally%20support%20transport%20mechanisms%20of%20RC%20transit.%20In%20addition%2C%20our%20tracking%20analysis%20indicates%20that%20Golgi%20are%20actively%20transported%20to%20the%20oocyte%20rather%20than%20diffusing.%20We%20observed%20that%20RC%20transit%20is%20microtubule-based%20and%20mediated%20at%20least%20by%20dynein.%20Finally%2C%20we%20show%20that%20actin%20networks%20may%20be%20involved%20in%20RC%20crossing%20through%20a%20myosin%20II%20step%20process%2C%20as%20well%20as%20in%20dispatching%20Golgi%20units%20inside%20the%20oocyte%20subcompartments.%22%2C%22date%22%3A%222009-01%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1091%5C%2Fmbc.e08-04-0360%22%2C%22ISSN%22%3A%221059-1524%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.molbiolcell.org%5C%2Fdoi%5C%2F10.1091%5C%2Fmbc.e08-04-0360%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A14%3A03Z%22%7D%7D%2C%7B%22key%22%3A%225BHF3Z2E%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Gervais%20et%20al.%22%2C%22parsedDate%22%3A%222008-12-01%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BGervais%2C%20L.%2C%20Claret%2C%20S.%2C%20Januschke%2C%20J.%2C%20Roth%2C%20S.%2C%20%26amp%3B%20Guichet%2C%20A.%20%282008%29.%20PIP5K-dependent%20production%20of%20PIP2%20sustains%20microtubule%20organization%20to%20establish%20polarized%20transport%20in%20the%20Drosophila%20oocyte.%20%26lt%3Bi%26gt%3BDevelopment%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B135%26lt%3B%5C%2Fi%26gt%3B%2823%29%2C%203829%26%23×2013%3B3838.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fdev.029009%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fdev.029009%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22PIP5K-dependent%20production%20of%20PIP2%20sustains%20microtubule%20organization%20to%20establish%20polarized%20transport%20in%20the%20Drosophila%20oocyte%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Louis%22%2C%22lastName%22%3A%22Gervais%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sandra%22%2C%22lastName%22%3A%22Claret%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jens%22%2C%22lastName%22%3A%22Januschke%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Siegfried%22%2C%22lastName%22%3A%22Roth%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%5D%2C%22abstractNote%22%3A%22The%20attachment%20of%20the%20cytoskeleton%20to%20the%20plasma%20membrane%20is%20crucial%20in%20controlling%20the%20polarized%20transport%20of%20cell-fate-determining%20molecules.%20Attachment%20involves%20adaptor%20molecules%2C%20which%20have%20the%20capacity%20to%20bind%20to%20both%20the%20plasma%20membrane%20and%20elements%20of%20the%20cytoskeleton%2C%20such%20as%20microtubules%20and%20actin%20filaments.%20Using%20the%20Drosophila%20oocyte%20as%20a%20model%20system%2C%20we%20show%20that%20the%20type%20I%20phosphatidylinositol%204-phosphate%205-kinase%20%28PIP5K%29%2CSkittles%2C%20is%20necessary%20to%20sustain%20the%20organization%20of%20microtubules%20and%20actin%20cytoskeleton%20required%20for%20the%20asymmetric%20transport%20of%20oskar%2C%20bicoidand%20gurken%20mRNAs%20and%20thereby%20controls%20the%20establishment%20of%20cell%20polarity.%20We%20show%20that%20Skittles%20function%20is%20crucial%20to%20synthesize%20and%20maintain%20phosphatidylinositol%204%2C5%20bisphosphate%20%28PIP2%29%20at%20the%20plasma%20membrane%20in%20the%20oocyte.%20Reduction%20of%20Skittles%20activity%20impairs%20activation%20at%20the%20plasma%20membrane%20of%20Moesin%2C%20a%20member%20of%20the%20ERM%20family%20known%20to%20link%20the%20plasma%20membrane%20to%20the%20actin-based%20cytoskeleton.%20Furthermore%2C%20we%20provide%20evidence%20that%20Skittles%2C%20by%20controlling%20the%20localization%20of%20Bazooka%2C%20Par-1%20and%20Lgl%2C%20but%20not%20Lkb1%2C%20to%20the%20cell%20membrane%2C%20regulates%20PAR%20polarity%20proteins%20and%20the%20maintenance%20of%20specific%20cortical%20domains%20along%20the%20anteroposterior%20axis.%22%2C%22date%22%3A%222008-12-01%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1242%5C%2Fdev.029009%22%2C%22ISSN%22%3A%220950-1991%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fdev.029009%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A26%3A08Z%22%7D%7D%2C%7B%22key%22%3A%22BUDB9JAQ%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Januschke%20et%20al.%22%2C%22parsedDate%22%3A%222007-10-01%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BJanuschke%2C%20J.%2C%20Nicolas%2C%20E.%2C%20Compagnon%2C%20J.%2C%20Formstecher%2C%20E.%2C%20Goud%2C%20B.%2C%20%26amp%3B%20Guichet%2C%20A.%20%282007%29.%20Rab6%20and%20the%20secretory%20pathway%20affect%20oocyte%20polarity%20in%20Drosophila.%20%26lt%3Bi%26gt%3BDevelopment%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B134%26lt%3B%5C%2Fi%26gt%3B%2819%29%2C%203419%26%23×2013%3B3425.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fdev.008078%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fdev.008078%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Rab6%20and%20the%20secretory%20pathway%20affect%20oocyte%20polarity%20in%20Drosophila%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jens%22%2C%22lastName%22%3A%22Januschke%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Emmanuelle%22%2C%22lastName%22%3A%22Nicolas%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Julien%22%2C%22lastName%22%3A%22Compagnon%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Etienne%22%2C%22lastName%22%3A%22Formstecher%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Bruno%22%2C%22lastName%22%3A%22Goud%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%5D%2C%22abstractNote%22%3A%22The%20Drosophila%20oocyte%20is%20a%20highly%20polarized%20cell.%20Secretion%20occurs%20towards%20restricted%20neighboring%20cells%20and%20asymmetric%20transport%20controls%20the%20localization%20of%20several%20mRNAs%20to%20distinct%20cortical%20compartments.%20Here%2C%20we%20describe%20a%20role%20for%20the%20Drosophila%20ortholog%20of%20the%20Rab6%20GTPase%2CDrab6%2C%20in%20establishing%20cell%20polarity%20during%20oogenesis.%20We%20found%20that%20Drab6%20localizes%20to%20Golgi%20and%20Golgi-derived%20membranes%20and%20interacts%20with%20BicD.%20We%20also%20provide%20evidence%20that%20Drab6%20and%20BicD%20function%20together%20to%20ensure%20the%20correct%20delivery%20of%20secretory%20pathway%20components%2C%20such%20as%20the%20TGF%5Cu03b1homolog%20Gurken%2C%20to%20the%20plasma%20membrane.%20Moreover%2C%20in%20the%20absence%20of%20Drab6%2C%20osk%20mRNA%20localization%20and%20the%20organization%20of%20microtubule%20plus-ends%20at%20the%20posterior%20of%20the%20oocyte%20were%20both%20severely%20affected.%20Our%20results%20point%20to%20a%20possible%20connection%20between%20Rab%20protein-mediated%20secretion%2C%20organization%20of%20the%20cytoskeleton%20and%20mRNA%20transport.%22%2C%22date%22%3A%222007-10-01%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1242%5C%2Fdev.008078%22%2C%22ISSN%22%3A%220950-1991%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fdev.008078%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A26%3A24Z%22%7D%7D%2C%7B%22key%22%3A%22Q8RI564X%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22GUICHET%22%2C%22parsedDate%22%3A%222007%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BGUICHET%2C%20A.%20%282007%29.%20Mise%20en%20place%20de%20la%20polarit%26%23xE9%3B%20au%20sein%20de%20l%26%23×2019%3Bovocyte%20de%20drosophile%26%23x202F%3B%3A%20%28R%29%26%23xE9%3Bvolution%20du%20d%26%23xE9%3Bveloppement.%20%26lt%3Bi%26gt%3BMise%20En%20Place%20de%20La%20Polarit%26%23xE9%3B%20Au%20Sein%20de%20l%26%23×2019%3Bovocyte%20de%20Drosophile%26%23x202F%3B%3A%20%28R%29%26%23xC9%3Bvolution%20Du%20D%26%23xE9%3Bveloppement%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B281%26lt%3B%5C%2Fi%26gt%3B%2C%2024%26%23×2013%3B27.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Mise%20en%20place%20de%20la%20polarit%5Cu00e9%20au%20sein%20de%20l%27ovocyte%20de%20drosophile%20%3A%20%28R%29%5Cu00e9volution%20du%20d%5Cu00e9veloppement%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22GUICHET%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222007%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22ISSN%22%3A%220294-3506%22%2C%22url%22%3A%22%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A15%3A31Z%22%7D%7D%2C%7B%22key%22%3A%227UUM8KHR%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Januschke%20et%20al.%22%2C%22parsedDate%22%3A%222006-01-01%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BJanuschke%2C%20J.%2C%20Gervais%2C%20L.%2C%20Gillet%2C%20L.%2C%20Keryer%2C%20G.%2C%20Bornens%2C%20M.%2C%20%26amp%3B%20Guichet%2C%20A.%20%282006%29.%20The%20centrosome-nucleus%20complex%20and%20microtubule%20organization%20in%20the%20Drosophila%20oocyte.%20%26lt%3Bi%26gt%3BDevelopment%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B133%26lt%3B%5C%2Fi%26gt%3B%281%29%2C%20129%26%23×2013%3B139.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fdev.02179%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fdev.02179%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22The%20centrosome-nucleus%20complex%20and%20microtubule%20organization%20in%20the%20Drosophila%20oocyte%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jens%22%2C%22lastName%22%3A%22Januschke%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Louis%22%2C%22lastName%22%3A%22Gervais%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Laurent%22%2C%22lastName%22%3A%22Gillet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Guy%22%2C%22lastName%22%3A%22Keryer%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Michel%22%2C%22lastName%22%3A%22Bornens%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%5D%2C%22abstractNote%22%3A%22Molecular%20motors%20transport%20the%20axis-determining%20mRNAs%20oskar%2Cbicoid%20and%20gurken%20along%20microtubules%20%28MTs%29%20in%20the%20Drosophila%20oocyte.%20However%2C%20it%20remains%20unclear%20how%20the%20underlying%20MT%20network%20is%20organized%20and%20how%20this%20transport%20takes%20place.%20We%20have%20identified%20a%20centriole-containing%20centrosome%20close%20to%20the%20oocyte%20nucleus.%20Remarkably%2C%20the%20centrosomal%20components%2C%20%5Cu03b3-tubulin%20and%20Drosophilapericentrin-like%20protein%20also%20strongly%20accumulate%20at%20the%20periphery%20of%20this%20nucleus.%20MT%20polymerization%20after%20cold-induced%20disassembly%20in%20wild%20type%20and%20in%20gurken%20mutants%20suggests%20that%20in%20the%20oocyte%20the%20centrosome-nucleus%20complex%20is%20an%20active%20center%20of%20MT%20polymerization.%20We%20further%20report%20that%20the%20MT%20network%20comprises%20two%20perpendicular%20MT%20subsets%20that%20undergo%20dynamic%20rearrangements%20during%20oogenesis.%20This%20MT%20reorganization%20parallels%20the%20successive%20steps%20in%20localization%20of%20gurken%20and%20oskar%20mRNAs.%20We%20propose%20that%20in%20addition%20to%20a%20highly%20polarized%20microtubule%20scaffold%20specified%20by%20the%20cortex%20oocyte%2C%20the%20repositioning%20of%20the%20nucleus%20and%20its%20tightly%20associated%20centrosome%20could%20control%20MT%20reorganization%20and%2C%20hence%2Coocyte%20polarization.%22%2C%22date%22%3A%222006-01-01%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1242%5C%2Fdev.02179%22%2C%22ISSN%22%3A%220950-1991%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fdev.02179%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A15%3A49Z%22%7D%7D%2C%7B%22key%22%3A%22B8NWJQXA%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Januschke%20et%20al.%22%2C%22parsedDate%22%3A%222002-12-10%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BJanuschke%2C%20J.%2C%20Gervais%2C%20L.%2C%20Dass%2C%20S.%2C%20Kaltschmidt%2C%20J.%20A.%2C%20Lopez-Schier%2C%20H.%2C%20Johnston%2C%20D.%20St.%2C%20Brand%2C%20A.%20H.%2C%20Roth%2C%20S.%2C%20%26amp%3B%20Guichet%2C%20A.%20%282002%29.%20Polar%20Transport%20in%20the%20%26lt%3Bi%26gt%3BDrosophila%26lt%3B%5C%2Fi%26gt%3B%20Oocyte%20Requires%20Dynein%20and%20Kinesin%20I%20Cooperation.%20%26lt%3Bi%26gt%3BCurrent%20Biology%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B12%26lt%3B%5C%2Fi%26gt%3B%2823%29%2C%201971%26%23×2013%3B1981.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2FS0960-9822%2802%2901302-7%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2FS0960-9822%2802%2901302-7%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Polar%20Transport%20in%20the%20%3Ci%3EDrosophila%3C%5C%2Fi%3E%20Oocyte%20Requires%20Dynein%20and%20Kinesin%20I%20Cooperation%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jens%22%2C%22lastName%22%3A%22Januschke%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Louis%22%2C%22lastName%22%3A%22Gervais%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sajith%22%2C%22lastName%22%3A%22Dass%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Julia%20A.%22%2C%22lastName%22%3A%22Kaltschmidt%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Hernan%22%2C%22lastName%22%3A%22Lopez-Schier%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Daniel%20St.%22%2C%22lastName%22%3A%22Johnston%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Andrea%20H.%22%2C%22lastName%22%3A%22Brand%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Siegfried%22%2C%22lastName%22%3A%22Roth%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%5D%2C%22abstractNote%22%3A%22Background%3A%20The%20cytoskeleton%20and%20associated%20motors%20play%20an%20important%20role%20in%20the%20establishment%20of%20intracellular%20polarity.%20Microtubule-based%20transport%20is%20required%20in%20many%20cell%20types%20for%20the%20asymmetric%20localization%20of%20mRNAs%20and%20organelles.%20A%20striking%20example%20is%20the%20Drosophila%20oocyte%2C%20where%20microtubule-dependent%20processes%20govern%20the%20asymmetric%20positioning%20of%20the%20nucleus%20and%20the%20localization%20to%20distinct%20cortical%20domains%20of%20mRNAs%20that%20function%20as%20cytoplasmic%20determinants.%20A%20conserved%20machinery%20for%20mRNA%20localization%20and%20nuclear%20positioning%20involving%20cytoplasmic%20Dynein%20has%20been%20postulated%3B%20however%2C%20the%20precise%20role%20of%20plus-%20and%20minus%20end-directed%20microtubule-based%20transport%20in%20axis%20formation%20is%20not%20yet%20understood.%20Results%3A%20Here%2C%20we%20show%20that%20mRNA%20localization%20and%20nuclear%20positioning%20at%20mid-oogenesis%20depend%20on%20two%20motor%20proteins%2C%20cytoplasmic%20Dynein%20and%20Kinesin%20I.%20Both%20of%20these%20microtubule%20motors%20cooperate%20in%20the%20polar%20transport%20of%20bicoid%20and%20gurken%20mRNAs%20to%20their%20respective%20cortical%20domains.%20In%20contrast%2C%20Kinesin%20I-mediated%20transport%20of%20oskar%20to%20the%20posterior%20pole%20appears%20to%20be%20independent%20of%20Dynein.%20Beside%20their%20roles%20in%20RNA%20transport%2C%20both%20motors%20are%20involved%20in%20nuclear%20positioning%20and%20in%20exocytosis%20of%20Gurken%20protein.%20Dynein-Dynactin%20complexes%20accumulate%20at%20two%20sites%20within%20the%20oocyte%3A%20around%20the%20nucleus%20in%20a%20microtubule-independent%20manner%20and%20at%20the%20posterior%20pole%20through%20Kinesin-mediated%20transport.%20Conclusion%3A%20The%20microtubule%20motors%20cytoplasmic%20Dynein%20and%20Kinesin%20I%2C%20by%20driving%20transport%20to%20opposing%20microtubule%20ends%2C%20function%20in%20concert%20to%20establish%20intracellular%20polarity%20within%20the%20Drosophila%20oocyte.%20Furthermore%2C%20Kinesin-dependent%20localization%20of%20Dynein%20suggests%20that%20both%20motors%20are%20components%20of%20the%20same%20complex%20and%20therefore%20might%20cooperate%20in%20recycling%20each%20other%20to%20the%20opposite%20microtubule%20pole.%22%2C%22date%22%3A%222002-12-10%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1016%5C%2FS0960-9822%2802%2901302-7%22%2C%22ISSN%22%3A%220960-9822%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.sciencedirect.com%5C%2Fscience%5C%2Farticle%5C%2Fpii%5C%2FS0960982202013027%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A16%3A07Z%22%7D%7D%2C%7B%22key%22%3A%226VPCWYWU%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Tazuke%20et%20al.%22%2C%22parsedDate%22%3A%222002-05-15%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BTazuke%2C%20S.%20I.%2C%20Schulz%2C%20C.%2C%20Gilboa%2C%20L.%2C%20Fogarty%2C%20M.%2C%20Mahowald%2C%20A.%20P.%2C%20Guichet%2C%20A.%2C%20Ephrussi%2C%20A.%2C%20Wood%2C%20C.%20G.%2C%20Lehmann%2C%20R.%2C%20%26amp%3B%20Fuller%2C%20M.%20T.%20%282002%29.%20A%20germline-specific%20gap%20junction%20protein%20required%20for%20survival%20of%20differentiating%20early%20germ%20cells.%20%26lt%3Bi%26gt%3BDevelopment%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B129%26lt%3B%5C%2Fi%26gt%3B%2810%29%2C%202529%26%23×2013%3B2539.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fdev.129.10.2529%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fdev.129.10.2529%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22A%20germline-specific%20gap%20junction%20protein%20required%20for%20survival%20of%20differentiating%20early%20germ%20cells%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Salli%20I.%22%2C%22lastName%22%3A%22Tazuke%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Cordula%22%2C%22lastName%22%3A%22Schulz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Lilach%22%2C%22lastName%22%3A%22Gilboa%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mignon%22%2C%22lastName%22%3A%22Fogarty%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anthony%20P.%22%2C%22lastName%22%3A%22Mahowald%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anne%22%2C%22lastName%22%3A%22Ephrussi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Cricket%20G.%22%2C%22lastName%22%3A%22Wood%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ruth%22%2C%22lastName%22%3A%22Lehmann%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Margaret%20T.%22%2C%22lastName%22%3A%22Fuller%22%7D%5D%2C%22abstractNote%22%3A%22Germ%20cells%20require%20intimate%20associations%20and%20signals%20from%20the%20surrounding%20somatic%20cells%20throughout%20gametogenesis.%20The%20zero%20population%20growth%20%28zpg%29%20locus%20of%20Drosophila%20encodes%20a%20germline-specific%20gap%20junction%20protein%2C%20Innexin%204%2C%20that%20is%20required%20for%20survival%20of%20differentiating%20early%20germ%20cells%20during%20gametogenesis%20in%20both%20sexes.%20Animals%20with%20a%20null%20mutation%20in%20zpg%20are%20viable%20but%20sterile%20and%20have%20tiny%20gonads.%20Adult%20zpg-null%20gonads%20contain%20small%20numbers%20of%20early%20germ%20cells%2C%20resembling%20stem%20cells%20or%20early%20spermatogonia%20or%20oogonia%2C%20but%20lack%20later%20stages%20of%20germ%20cell%20differentiation.%20In%20the%20male%2C%20Zpg%20protein%20localizes%20to%20the%20surface%20of%20spermatogonia%2C%20primarily%20on%20the%20sides%20adjacent%20to%20the%20somatic%20cyst%20cells.%20In%20the%20female%2C%20Zpg%20protein%20localizes%20to%20germ%20cell%20surfaces%2C%20both%20those%20adjacent%20to%20surrounding%20somatic%20cells%20and%20those%20adjacent%20to%20other%20germ%20cells.%20We%20propose%20that%20Zpg-containing%20gap%20junctional%20hemichannels%20in%20the%20germ%20cell%20plasma%20membrane%20may%20connect%20with%20hemichannels%20made%20of%20other%20innexin%20isoforms%20on%20adjacent%20somatic%20cells.%20Gap%20junctional%20intercellular%20communication%20via%20these%20channels%20may%20mediate%20passage%20of%20crucial%20small%20molecules%20or%20signals%20between%20germline%20and%20somatic%20support%20cells%20required%20for%20survival%20and%20differentiation%20of%20early%20germ%20cells%20in%20both%20sexes.%22%2C%22date%22%3A%222002-05-15%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1242%5C%2Fdev.129.10.2529%22%2C%22ISSN%22%3A%220950-1991%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fdev.129.10.2529%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A17%3A17Z%22%7D%7D%2C%7B%22key%22%3A%22UM5LR5E8%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Guichet%20et%20al.%22%2C%22parsedDate%22%3A%222002-04%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BGuichet%2C%20A.%2C%20Wucherpfennig%2C%20T.%2C%20Dudu%2C%20V.%2C%20Etter%2C%20S.%2C%20Wilsch%26%23×2010%3BBr%26%23xE4%3Buniger%2C%20M.%2C%20Hellwig%2C%20A.%2C%20Gonz%26%23xE1%3Blez%26%23×2010%3BGait%26%23xE1%3Bn%2C%20M.%2C%20Huttner%2C%20W.%20B.%2C%20%26amp%3B%20Schmidt%2C%20A.%20A.%20%282002%29.%20Essential%20role%20of%20endophilin%20A%20in%20synaptic%20vesicle%20budding%20at%20the%20Drosophila%20neuromuscular%20junction.%20%26lt%3Bi%26gt%3BThe%20EMBO%20Journal%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B21%26lt%3B%5C%2Fi%26gt%3B%287%29%2C%201661%26%23×2013%3B1672.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1093%5C%2Femboj%5C%2F21.7.1661%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1093%5C%2Femboj%5C%2F21.7.1661%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Essential%20role%20of%20endophilin%20A%20in%20synaptic%20vesicle%20budding%20at%20the%20Drosophila%20neuromuscular%20junction%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Tanja%22%2C%22lastName%22%3A%22Wucherpfennig%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Veronica%22%2C%22lastName%22%3A%22Dudu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sylvain%22%2C%22lastName%22%3A%22Etter%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Michaela%22%2C%22lastName%22%3A%22Wilsch%5Cu2010Br%5Cu00e4uniger%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Andrea%22%2C%22lastName%22%3A%22Hellwig%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Marcos%22%2C%22lastName%22%3A%22Gonz%5Cu00e1lez%5Cu2010Gait%5Cu00e1n%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Wieland%20B.%22%2C%22lastName%22%3A%22Huttner%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anne%20A.%22%2C%22lastName%22%3A%22Schmidt%22%7D%5D%2C%22abstractNote%22%3A%22We%20characterized%20Drosophila%20endophilin%20A%20%28D%5Cu2010endoA%29%2C%20and%20generated%20and%20analysed%20D%5Cu2010endoA%20mutants.%20Like%20its%20mammalian%20homologue%2C%20D%5Cu2010endoA%20exhibits%20lysophosphatidic%20acid%20acyl%20transferase%20activity%20and%20contains%20a%20functional%20SH3%20domain.%20D%5Cu2010endoA%20is%20recruited%20to%20the%20sites%20of%20endocytosis%2C%20as%20revealed%20by%20immunocytochemistry%20of%20the%20neuromuscular%20junction%20%28NMJ%29%20of%20mutant%20L3%20larvae%20carrying%20the%20temperature%5Cu2010sensitive%20allele%20of%20dynamin%2C%20shibire.%20D%5Cu2010endoA%20null%20mutants%20show%20severe%20defects%20in%20motility%20and%20die%20at%20the%20early%20L2%20larval%20stage.%20Mutants%20with%20reduced%20D%5Cu2010endoA%20levels%20exhibit%20a%20range%20of%20defects%20of%20synaptic%20vesicle%20endocytosis%2C%20as%20observed%20at%20L3%20larvae%20NMJs%20using%20FM1%5Cu201043%20uptake%20and%20electron%20microscopy.%20NMJs%20with%20an%20almost%20complete%20loss%20of%20synaptic%20vesicles%20did%20not%20show%20an%20accumulation%20of%20intermediates%20of%20the%20budding%20process%2C%20whereas%20NMJs%20with%20only%20slightly%20reduced%20levels%20of%20synaptic%20vesicles%20showed%20a%20striking%20increase%20in%20early%5Cu2010stage%2C%20but%20not%20late%5Cu2010stage%2C%20budding%20intermediates%20at%20the%20plasma%20membrane.%20Together%20with%20results%20of%20previous%20studies%2C%20these%20observations%20indicate%20that%20endophilin%20A%20is%20essential%20for%20synaptic%20vesicle%20endocytosis%2C%20being%20required%20from%20the%20onset%20of%20budding%20until%20fission.%22%2C%22date%22%3A%222002-04%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1093%5C%2Femboj%5C%2F21.7.1661%22%2C%22ISSN%22%3A%220261-4189%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.embopress.org%5C%2Fdoi%5C%2Ffull%5C%2F10.1093%5C%2Femboj%5C%2F21.7.1661%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A22%3A56Z%22%7D%7D%2C%7B%22key%22%3A%225TQQUKJL%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Ozon%20et%20al.%22%2C%22parsedDate%22%3A%222002-02%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BOzon%2C%20S.%2C%20Guichet%2C%20A.%2C%20Gavet%2C%20O.%2C%20Roth%2C%20S.%2C%20%26amp%3B%20Sobel%2C%20A.%20%282002%29.%20Drosophila%20Stathmin%3A%20A%20Microtubule-destabilizing%20Factor%20Involved%20in%20Nervous%20System%20Formation.%20%26lt%3Bi%26gt%3BMolecular%20Biology%20of%20the%20Cell%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B13%26lt%3B%5C%2Fi%26gt%3B%282%29%2C%20698%26%23×2013%3B710.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1091%5C%2Fmbc.01-07-0362%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1091%5C%2Fmbc.01-07-0362%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Drosophila%20Stathmin%3A%20A%20Microtubule-destabilizing%20Factor%20Involved%20in%20Nervous%20System%20Formation%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sylvie%22%2C%22lastName%22%3A%22Ozon%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Olivier%22%2C%22lastName%22%3A%22Gavet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Siegfried%22%2C%22lastName%22%3A%22Roth%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Andr%5Cu00e9%22%2C%22lastName%22%3A%22Sobel%22%7D%5D%2C%22abstractNote%22%3A%22Stathmin%20is%20a%20ubiquitous%20regulatory%20phosphoprotein%2C%20the%20generic%20element%20of%20a%20family%20of%20neural%20phosphoproteins%20in%20vertebrates%20that%20possess%20the%20capacity%20to%20bind%20tubulin%20and%20interfere%20with%20microtubule%20dynamics.%20Although%20stathmin%20and%20the%20other%20proteins%20of%20the%20family%20have%20been%20associated%20with%20numerous%20cell%20regulations%2C%20their%20biological%20roles%20remain%20elusive%2C%20as%20in%20particular%20inactivation%20of%20the%20stathmin%20gene%20in%20the%20mouse%20resulted%20in%20no%20clear%20deleterious%20phenotype.%20We%20identified%20stathmin%20phosphoproteins%20inDrosophila%2C%20encoded%20by%20a%20unique%20gene%20sharing%20the%20intron%5C%2Fexon%20structure%20of%20the%20vertebrate%20stathmin%20andstathminfamily%20genes.%20They%20interfere%20with%20microtubule%20assembly%20in%20vitro%2C%20and%20in%20vivo%20when%20expressed%20in%20HeLa%20cells.%20Drosophila%20stathmin%20expression%20is%20regulated%20during%20embryogenesis%3A%20it%20is%20high%20in%20the%20migrating%20germ%20cells%20and%20in%20the%20central%20and%20peripheral%20nervous%20systems%2C%20a%20pattern%20resembling%20that%20of%20mammalian%20stathmin.%20Furthermore%2C%20RNA%20interference%20inactivation%20ofDrosophila%20stathmin%20expression%20resulted%20in%20germ%20cell%20migration%20arrest%20at%20stage%2014.%20It%20also%20induced%20important%20anomalies%20in%20nervous%20system%20development%2C%20such%20as%20loss%20of%20commissures%20and%20longitudinal%20connectives%20in%20the%20ventral%20cord%2C%20or%20abnormal%20chordotonal%20neuron%20organization.%20In%20conclusion%2C%20a%20single%20Drosophilagene%20encodes%20phosphoproteins%20homologous%20to%20the%20entire%20vertebrate%20stathmin%20family.%20We%20demonstrate%20for%20the%20first%20time%20their%20direct%20involvement%20in%20major%20biological%20processes%20such%20as%20development%20of%20the%20reproductive%20and%20nervous%20systems.%22%2C%22date%22%3A%222002-02%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1091%5C%2Fmbc.01-07-0362%22%2C%22ISSN%22%3A%221059-1524%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.molbiolcell.org%5C%2Fdoi%5C%2F10.1091%5C%2Fmbc.01-07-0362%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A22%3A29Z%22%7D%7D%2C%7B%22key%22%3A%22V539CXKB%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Guichet%20et%20al.%22%2C%22parsedDate%22%3A%222001-09-01%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BGuichet%2C%20A.%2C%20Peri%2C%20F.%2C%20%26amp%3B%20Roth%2C%20S.%20%282001%29.%20Stable%20Anterior%20Anchoring%20of%20the%20Oocyte%20Nucleus%20Is%20Required%20to%20Establish%20Dorsoventral%20Polarity%20of%20the%20%26lt%3Bi%26gt%3BDrosophila%26lt%3B%5C%2Fi%26gt%3B%20Egg.%20%26lt%3Bi%26gt%3BDevelopmental%20Biology%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B237%26lt%3B%5C%2Fi%26gt%3B%281%29%2C%2093%26%23×2013%3B106.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1006%5C%2Fdbio.2001.0354%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1006%5C%2Fdbio.2001.0354%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Stable%20Anterior%20Anchoring%20of%20the%20Oocyte%20Nucleus%20Is%20Required%20to%20Establish%20Dorsoventral%20Polarity%20of%20the%20%3Ci%3EDrosophila%3C%5C%2Fi%3E%20Egg%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Francesca%22%2C%22lastName%22%3A%22Peri%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Siegfried%22%2C%22lastName%22%3A%22Roth%22%7D%5D%2C%22abstractNote%22%3A%22In%20Drosophila%2C%20dorsoventral%20polarity%20is%20established%20by%20the%20asymmetric%20positioning%20of%20the%20oocyte%20nucleus.%20In%20egg%20chambers%20mutant%20for%20cap%20%5Cu2018n%5Cu2019%20collar%2C%20the%20oocyte%20nucleus%20migrates%20correctly%20from%20a%20posterior%20to%20an%20anterior%5Cu2013dorsal%20position%20where%20it%20remains%20during%20stage%209%20of%20oogenesis.%20However%2C%20at%20the%20end%20of%20stage%209%2C%20the%20nucleus%20leaves%20its%20anterior%20position%20and%20migrates%20towards%20the%20posterior%20pole.%20The%20mislocalisation%20of%20the%20nucleus%20is%20accompanied%20by%20changes%20in%20the%20microtubule%20network%20and%20a%20failure%20to%20maintain%20bicoid%20and%20oskar%20mRNAs%20at%20the%20anterior%20and%20posterior%20poles%2C%20respectively.%20gurken%20mRNA%20associates%20with%20the%20oocyte%20nucleus%20in%20cap%20%5Cu2018n%5Cu2019%20collar%20mutants%20and%20initially%20the%20local%20secretion%20of%20Gurken%20protein%20activates%20the%20Drosophila%20EGF%20receptor%20in%20the%20overlying%20dorsal%20follicle%20cells.%20However%2C%20despite%20the%20presence%20of%20spatially%20correct%20Grk%20signalling%20during%20stage%209%2C%20eggs%20laid%20by%20cap%20%5Cu2018n%5Cu2019%20collar%20females%20lack%20dorsoventral%20polarity.%20cap%20%5Cu2018n%5Cu2019%20collar%20mutants%2C%20therefore%2C%20allow%20for%20the%20study%20of%20the%20influence%20of%20Grk%20signal%20duration%20on%20DV%20patterning%20in%20the%20follicular%20epithelium.%22%2C%22date%22%3A%222001-09-01%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1006%5C%2Fdbio.2001.0354%22%2C%22ISSN%22%3A%220012-1606%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.sciencedirect.com%5C%2Fscience%5C%2Farticle%5C%2Fpii%5C%2FS0012160601903549%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A26%3A37Z%22%7D%7D%2C%7B%22key%22%3A%22ZWNNFE8H%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Jim%5Cu00e9nez%20et%20al.%22%2C%22parsedDate%22%3A%222000-01-15%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BJim%26%23xE9%3Bnez%2C%20G.%2C%20Guichet%2C%20A.%2C%20Ephrussi%2C%20A.%2C%20%26amp%3B%20Casanova%2C%20J.%20%282000%29.%20Relief%20of%20gene%20repression%20by%20torso%20RTK%20signaling%3A%20role%20of%20capicua%20in%20Drosophila%20terminal%20and%20dorsoventral%20patterning.%20%26lt%3Bi%26gt%3BGenes%20%26amp%3B%20Development%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B14%26lt%3B%5C%2Fi%26gt%3B%282%29%2C%20224%26%23×2013%3B231.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Relief%20of%20gene%20repression%20by%20torso%20RTK%20signaling%3A%20role%20of%20capicua%20in%20Drosophila%20terminal%20and%20dorsoventral%20patterning%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22G.%22%2C%22lastName%22%3A%22Jim%5Cu00e9nez%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22A.%22%2C%22lastName%22%3A%22Guichet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22A.%22%2C%22lastName%22%3A%22Ephrussi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J.%22%2C%22lastName%22%3A%22Casanova%22%7D%5D%2C%22abstractNote%22%3A%22Differentiation%20of%20the%20embryonic%20termini%20in%20Drosophila%20depends%20on%20signaling%20by%20the%20Tor%20RTK%2C%20which%20induces%20terminal%20gene%20expression%20by%20inactivating%20at%20the%20embryonic%20poles%20a%20uniformly%20distributed%20repressor%20activity%20that%20involves%20the%20Gro%20corepressor.%20Here%2C%20we%20identify%20a%20new%20gene%2C%20cic%2C%20that%20acts%20as%20a%20repressor%20of%20terminal%20genes%20regulated%20by%20the%20Tor%20pathway.%20cic%20also%20mediates%20repression%20along%20the%20dorsoventral%20axis%2C%20a%20process%20that%20requires%20the%20Dorsal%20morphogen%20and%20Gro%2C%20and%20which%20is%20also%20inhibited%20by%20Tor%20signaling%20at%20the%20termini.%20cic%20encodes%20an%20HMG-box%20transcription%20factor%20that%20interacts%20with%20Gro%20in%20vitro.%20We%20present%20evidence%20that%20Tor%20signaling%20regulates%20terminal%20patterning%20by%20inactivating%20Cic%20at%20the%20embryo%20poles.%20cic%20has%20been%20evolutionarily%20conserved%2C%20suggesting%20that%20Cic-like%20proteins%20may%20act%20as%20repressors%20regulated%20by%20RTK%20signaling%20in%20other%20organisms.%22%2C%22date%22%3A%222000-01-15%22%2C%22language%22%3A%22eng%22%2C%22DOI%22%3A%22%22%2C%22ISSN%22%3A%220890-9369%22%2C%22url%22%3A%22%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A18%3A11Z%22%7D%7D%2C%7B%22key%22%3A%22L87MN757%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Tomancak%20et%20al.%22%2C%22parsedDate%22%3A%221998-05-01%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BTomancak%2C%20P.%2C%20Guichet%2C%20A.%2C%20Zavorszky%2C%20P.%2C%20%26amp%3B%20Ephrussi%2C%20A.%20%281998%29.%20Oocyte%20polarity%20depends%20on%20regulation%20of%20gurken%20by%20Vasa.%20%26lt%3Bi%26gt%3BDevelopment%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B125%26lt%3B%5C%2Fi%26gt%3B%289%29%2C%201723%26%23×2013%3B1732.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fdev.125.9.1723%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fdev.125.9.1723%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Oocyte%20polarity%20depends%20on%20regulation%20of%20gurken%20by%20Vasa%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pavel%22%2C%22lastName%22%3A%22Tomancak%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Peter%22%2C%22lastName%22%3A%22Zavorszky%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anne%22%2C%22lastName%22%3A%22Ephrussi%22%7D%5D%2C%22abstractNote%22%3A%22Vasa%2C%20a%20DEAD%20box%20mRNA%20helicase%20similar%20to%20eIF4A%2C%20is%20involved%20in%20pole%20plasm%20assembly%20in%20the%20Drosophila%20oocyte%20and%20appears%20to%20regulate%20translation%20of%20oskar%20and%20nanos%20mRNAs.%20However%2C%20several%20vasa%20alleles%20exhibit%20a%20wide%20range%20of%20early%20oogenesis%20phenotypes.%20Here%20we%20report%20a%20detailed%20analysis%20of%20Vasa%20function%20during%20early%20oogenesis%20using%20novel%20as%20well%20as%20previously%20identified%20hypomorphic%20vasa%20alleles.%20We%20find%20that%20vasa%20is%20required%20for%20the%20establishment%20of%20both%20anterior-posterior%20and%20dorsal-ventral%20polarity%20of%20the%20oocyte.%20The%20polarity%20defects%20of%20vasa%20mutants%20appear%20to%20be%20caused%20by%20a%20reduction%20in%20the%20amount%20of%20Gurken%20protein%20at%20stages%20of%20oogenesis%20critical%20for%20the%20establishment%20of%20polarity.%20Vasa%20is%20required%20for%20translation%20of%20gurken%20mRNA%20during%20early%20oogenesis%20and%20for%20achieving%20wild-type%20levels%20of%20gurken%20mRNA%20expression%20later%20in%20oogenesis.%20A%20variety%20of%20early%20oogenesis%20phenotypes%20observed%20in%20vasa%20ovaries%2C%20which%20cannot%20be%20attributed%20to%20the%20defect%20in%20gurken%20expression%2C%20suggest%20that%20vasa%20also%20affects%20expression%20of%20other%20mRNAs.%22%2C%22date%22%3A%221998-05-01%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1242%5C%2Fdev.125.9.1723%22%2C%22ISSN%22%3A%220950-1991%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fdev.125.9.1723%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A24%3A30Z%22%7D%7D%2C%7B%22key%22%3A%22U7F98QZK%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Florence%20et%20al.%22%2C%22parsedDate%22%3A%221997-02-15%22%2C%22numChildren%22%3A2%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BFlorence%2C%20B.%2C%20Guichet%2C%20A.%2C%20Ephrussi%2C%20A.%2C%20%26amp%3B%20Laughon%2C%20A.%20%281997%29.%20Ftz-F1%20is%20a%20cofactor%20in%20Ftz%20activation%20of%20the%20Drosophila%20engrailed%20gene.%20%26lt%3Bi%26gt%3BDevelopment%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B124%26lt%3B%5C%2Fi%26gt%3B%284%29%2C%20839%26%23×2013%3B847.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fdev.124.4.839%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fdev.124.4.839%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Ftz-F1%20is%20a%20cofactor%20in%20Ftz%20activation%20of%20the%20Drosophila%20engrailed%20gene%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Brian%22%2C%22lastName%22%3A%22Florence%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anne%22%2C%22lastName%22%3A%22Ephrussi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Allen%22%2C%22lastName%22%3A%22Laughon%22%7D%5D%2C%22abstractNote%22%3A%22The%20fushi%20tarazu%20pair-rule%20gene%20is%20required%20for%20the%20formation%20of%20alternating%20parasegmental%20boundaries%20in%20the%20Drosophila%20embryo.%20fushi%20tarazu%20encodes%20a%20homeodomain%20protein%20necessary%20for%20transcription%20of%20the%20engrailed%20gene%20in%20even-numbered%20parasegments.%20Here%20we%20report%20that%2C%20within%20an%20engrailed%20enhancer%2C%20adjacent%20and%20conserved%20binding%20sites%20for%20the%20Fushi%20tarazu%20protein%20and%20a%20cofactor%20are%20each%20necessary%2C%20and%20together%20sufficient%2C%20for%20transcriptional%20activation.%20Footprinting%20shows%20that%20the%20cofactor%20site%20can%20be%20bound%20specifically%20by%20Ftz-F1%2C%20a%20member%20of%20the%20nuclear%20receptor%20superfamily.%20Ftz-F1%20and%20the%20Fushi%20tarazu%20homeodomain%20bind%20the%20sites%20with%204-to%208-fold%20cooperativity%2C%20suggesting%20that%20direct%20contact%20between%20the%20two%20proteins%20may%20contribute%20to%20target%20recognition.%20Even%20parasegmental%20reporter%20expression%20is%20dependent%20on%20Fushi%20tarazu%20and%20maternal%20Ftz-F1%2C%20suggesting%20that%20these%20two%20proteins%20are%20indeed%20the%20factors%20that%20act%20upon%20the%20two%20sites%20in%20embryos.%20The%20two%20adjacent%20binding%20sites%20are%20also%20required%20for%20continued%20activity%20of%20the%20engrailed%20enhancer%20after%20Fushi%20tarazu%20protein%20is%20no%20longer%20detectable%2C%20including%20the%20period%20when%20engrailed%2C%20and%20the%20enhancer%2C%20become%20dependent%20upon%20wingless.%20We%20also%20report%20the%20existence%20of%20a%20separate%20negative%20regulatory%20element%20that%20apparently%20responds%20to%20odd-skipped.%22%2C%22date%22%3A%221997-02-15%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1242%5C%2Fdev.124.4.839%22%2C%22ISSN%22%3A%220950-1991%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1242%5C%2Fdev.124.4.839%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A23%3A31Z%22%7D%7D%2C%7B%22key%22%3A%22YPRW9UG3%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Guichet%20et%20al.%22%2C%22parsedDate%22%3A%221997-02%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BGuichet%2C%20A.%2C%20Copeland%2C%20J.%20W.%20R.%2C%20Erd%26%23xE9%3Blyi%2C%20M.%2C%20Hlousek%2C%20D.%2C%20Z%26%23xE1%3Bvorszky%2C%20P.%2C%20Ho%2C%20J.%2C%20Brown%2C%20S.%2C%20Percival-Smith%2C%20A.%2C%20Krause%2C%20H.%20M.%2C%20%26amp%3B%20Ephrussi%2C%20A.%20%281997%29.%20The%20nuclear%20receptor%20homologue%20Ftz-F1%20and%20the%20homeodomain%20protein%20Ftz%20are%20mutually%20dependent%20cofactors.%20%26lt%3Bi%26gt%3BNature%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B385%26lt%3B%5C%2Fi%26gt%3B%286616%29%2C%20548%26%23×2013%3B552.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1038%5C%2F385548a0%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1038%5C%2F385548a0%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22The%20nuclear%20receptor%20homologue%20Ftz-F1%20and%20the%20homeodomain%20protein%20Ftz%20are%20mutually%20dependent%20cofactors%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22John%20W.%20R.%22%2C%22lastName%22%3A%22Copeland%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mikl%5Cu00f3s%22%2C%22lastName%22%3A%22Erd%5Cu00e9lyi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Daniela%22%2C%22lastName%22%3A%22Hlousek%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22P%5Cu00e9ter%22%2C%22lastName%22%3A%22Z%5Cu00e1vorszky%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jacqueline%22%2C%22lastName%22%3A%22Ho%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Susan%22%2C%22lastName%22%3A%22Brown%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anthony%22%2C%22lastName%22%3A%22Percival-Smith%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Henry%20M.%22%2C%22lastName%22%3A%22Krause%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anne%22%2C%22lastName%22%3A%22Ephrussi%22%7D%5D%2C%22abstractNote%22%3A%22Nuclear%20hormone%20receptors%20and%20homeodomain%20proteins%20are%20two%20classes%20of%20transcription%20factor%20that%20regulate%20major%20developmental%20processes.%20Both%20depend%20on%20interactions%20with%20other%20proteins%20for%20specificity%20and%20activity.%20The%20Drosophila%20gene%20fushi%20tarazu%20%28ftz%29y%20which%20encodes%20a%20homeodomain%20protein1%20%28Ftz%29%2C%20is%20required%20zygo-tically%20for%20the%20formation%20of%20alternate%20segments%20in%20the%20developing%20embryo2.%20Here%20we%20show%20that%20the%20orphan%20nuclear%20receptor%20%5Cu03b1%5CtFtz-Fl%20%28ref.%203%29%2C%20which%20is%20deposited%20in%20the%20egg%20during%20oogenesis4%2C%20is%20an%20obligatory%20cofactor%20for%20Ftz.%20The%20two%20proteins%20interact%20specifically%20and%20directly%2C%20both%20in%20vitro%20and%20in%20vivo%2C%20through%20a%20conserved%20domain%20in%20the%20Ftz%20polypeptide.%20This%20interaction%20suggests%20that%20other%20nuclear%20receptor%5C%2Fhomeodomain%20protein%20interactions%20maybe%20important%20and%20common%20in%20developing%20organisms.%22%2C%22date%22%3A%221997-02%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1038%5C%2F385548a0%22%2C%22ISSN%22%3A%221476-4687%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.nature.com%5C%2Farticles%5C%2F385548a0%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A18%3A27Z%22%7D%7D%2C%7B%22key%22%3A%22NP3DMS5D%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Erd%5Cu00e9lyi%20et%20al.%22%2C%22parsedDate%22%3A%221995-10%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BErd%26%23xE9%3Blyi%2C%20M.%2C%20Michon%2C%20A.-M.%2C%20Guichet%2C%20A.%2C%20Glotzer%2C%20J.%20B.%2C%20%26amp%3B%20Ephrussi%2C%20A.%20%281995%29.%20Requirement%20for%20Drosophila%20cytoplasmic%20tropomyosin%20in%20oskar%20mRNA%20localization.%20%26lt%3Bi%26gt%3BNature%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B377%26lt%3B%5C%2Fi%26gt%3B%286549%29%2C%20524%26%23×2013%3B527.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1038%5C%2F377524a0%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1038%5C%2F377524a0%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Requirement%20for%20Drosophila%20cytoplasmic%20tropomyosin%20in%20oskar%20mRNA%20localization%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mikl%5Cu00f3s%22%2C%22lastName%22%3A%22Erd%5Cu00e9lyi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anne-Marie%22%2C%22lastName%22%3A%22Michon%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Guichet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jolanta%20Bogucka%22%2C%22lastName%22%3A%22Glotzer%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anne%22%2C%22lastName%22%3A%22Ephrussi%22%7D%5D%2C%22abstractNote%22%3A%22THE%20localization%20of%20oskar%20%28osk%29%20RNA%20to%20the%20posterior%20pole%20of%20the%20developing%20fruit%20fly%20%28Drosophila%29%20oocyte%20induces%20the%20assembly%20of%20pole%20plasm%2C%20causing%20development%20of%20the%20abdomen%20and%20germ%20line1%2C2.%20Failure%20to%20localize%20oskar%20RNA%20results%20in%20embryos%20that%20lack%20abdomen%20and%20germ%20cells.%20Conversely%2C%20mis-targeting%20of%20oskar%20RNA%20to%20the%20anterior%20of%20the%20oocyte%20causes%20formation%20of%20ectopic%20abdomen%20and%20germ%20cells%20at%20the%20anterior%20pole3.%20Maternal%20mutants%20that%20have%20reduced%20pole%20plasm%20activity%20produce%20sterile%20adults%20with%20normal%20abdominal%20development%2C%20suggesting%20that%20germ%20cells%20are%20more%20sensitive%20than%20abdomen%20to%20defects%20in%20pole%20plasm%20assembly4.%20Thus%20mutations%20in%20genes%20that%20reduce%20oskar%20RNA%20localization%20or%20activity%20can%20be%20recovered%20as%20viable%20sterile%20adults.%20In%20a%20screen%20for%20mutants%20defective%20in%20germ%20cell%20formation%2C%20we%20isolated%20nine%20alleles%20of%20the%20tropomyosin%20II%20gene5.%20Here%20we%20show%20that%20mutations%20in%20tropomyosin%20II%20%28TmII%29%20virtually%20abolish%20oskar%20RNA%20localization%20to%20the%20posterior%20pole%2C%20suggesting%20an%20involvement%20of%20the%20actin%20network%20in%20oskar%20RNA%20localization.%22%2C%22date%22%3A%221995-10%22%2C%22language%22%3A%22en%22%2C%22DOI%22%3A%2210.1038%5C%2F377524a0%22%2C%22ISSN%22%3A%221476-4687%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.nature.com%5C%2Farticles%5C%2F377524a0%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A19%3A01Z%22%7D%7D%2C%7B%22key%22%3A%22A5FWFECR%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3B%26lt%3Bi%26gt%3BOverexpression%20of%20Partner%20of%20Numb%20Induces%20Asymmetric%20Distribution%20of%20the%20PI4P%205-Kinase%20Skittles%20in%20Mitotic%20Sensory%20Organ%20Precursor%20Cells%20in%20Drosophila%20%7C%20PLOS%20ONE%26lt%3B%5C%2Fi%26gt%3B.%20%28n.d.%29.%20Retrieved%20February%2019%2C%202024%2C%20from%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fjournals.plos.org%5C%2Fplosone%5C%2Farticle%3Fid%3D10.1371%5C%2Fjournal.pone.0003072%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fjournals.plos.org%5C%2Fplosone%5C%2Farticle%3Fid%3D10.1371%5C%2Fjournal.pone.0003072%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Overexpression%20of%20Partner%20of%20Numb%20Induces%20Asymmetric%20Distribution%20of%20the%20PI4P%205-Kinase%20Skittles%20in%20Mitotic%20Sensory%20Organ%20Precursor%20Cells%20in%20Drosophila%20%7C%20PLOS%20ONE%22%2C%22creators%22%3A%5B%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fjournals.plos.org%5C%2Fplosone%5C%2Farticle%3Fid%3D10.1371%5C%2Fjournal.pone.0003072%22%2C%22language%22%3A%22%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A26%3A53Z%22%7D%7D%2C%7B%22key%22%3A%22NK3XS924%22%2C%22library%22%3A%7B%22id%22%3A2913254%7D%2C%22meta%22%3A%7B%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3B%26lt%3Bi%26gt%3BOverexpression%20of%20Partner%20of%20Numb%20Induces%20Asymmetric%20Distribution%20of%20the%20PI4P%205-Kinase%20Skittles%20in%20Mitotic%20Sensory%20Organ%20Precursor%20Cells%20in%20Drosophila%20%7C%20PLOS%20ONE%26lt%3B%5C%2Fi%26gt%3B.%20%28n.d.%29.%20Retrieved%20February%2019%2C%202024%2C%20from%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fjournals.plos.org%5C%2Fplosone%5C%2Farticle%3Fid%3D10.1371%5C%2Fjournal.pone.0003072%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fjournals.plos.org%5C%2Fplosone%5C%2Farticle%3Fid%3D10.1371%5C%2Fjournal.pone.0003072%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Overexpression%20of%20Partner%20of%20Numb%20Induces%20Asymmetric%20Distribution%20of%20the%20PI4P%205-Kinase%20Skittles%20in%20Mitotic%20Sensory%20Organ%20Precursor%20Cells%20in%20Drosophila%20%7C%20PLOS%20ONE%22%2C%22creators%22%3A%5B%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fjournals.plos.org%5C%2Fplosone%5C%2Farticle%3Fid%3D10.1371%5C%2Fjournal.pone.0003072%22%2C%22language%22%3A%22%22%2C%22collections%22%3A%5B%22UAHRRGCT%22%5D%2C%22dateModified%22%3A%222024-02-19T11%3A14%3A43Z%22%7D%7D%5D%7D
Guyot, L., Chahine, E., De Filippo, E., Lalanne, C., Brun, S., Hartmann, F. E., & Giraud, T. (2025). Sheltered load in fungal mating-type chromosomes revealed by fitness experiments.
Journal of Evolutionary Biology, voaf079.
https://doi.org/10.1093/jeb/voaf079
Mallart, C., Netter, S., Chalvet, F., Claret, S., Guichet, A., Montagne, J., Pret, A.-M., & Malartre, M. (2024). JAK-STAT-dependent contact between follicle cells and the oocyte controls Drosophila anterior-posterior polarity and germline development.
Nature Communications,
15(1), 1627.
https://doi.org/10.1038/s41467-024-45963-z
Lepesant, J.-A., Roland-Gosselin, F., Guillemet, C., Bernard, F., & Guichet, A. (2024). The Importance of the Position of the Nucleus in Drosophila Oocyte Development.
Cells,
13(2), 201.
https://doi.org/10.3390/cells13020201
Zhu, Z., Becam, I., Tovey, C. A., Elfarkouchi, A., Yen, E. C., Bernard, F., Guichet, A., & Conduit, P. T. (2023). Multifaceted modes of γ-tubulin complex recruitment and microtubule nucleation at mitotic centrosomes.
Journal of Cell Biology,
222(10), e202212043.
https://doi.org/10.1083/jcb.202212043
Loh, M., Bernard, F., & Guichet, A. (2023). Kinesin-1 promotes centrosome clustering and nuclear migration in the Drosophila oocyte.
Development, dev.201728.
https://doi.org/10.1242/dev.201728
Galenza, A., Moreno-Roman, P., Su, Y.-H., Acosta-Alvarez, L., Debec, A., Guichet, A., Knapp, J.-M., Kizilyaprak, C., Humbel, B. M., Kolotuev, I., & O’Brien, L. E. (2023). Basal stem cell progeny establish their apical surface in a junctional niche during turnover of an adult barrier epithelium.
Nature Cell Biology.
https://doi.org/10.1038/s41556-023-01116-w
Tovey, C. A., Tsuji, C., Egerton, A., Bernard, F., Guichet, A., de la Roche, M., & Conduit, P. T. (2021). Autoinhibition of Cnn binding to γ-TuRCs prevents ectopic microtubule nucleation and cell division defects.
The Journal of Cell Biology,
220(8), e202010020.
https://doi.org/10.1083/jcb.202010020
Loh, M., Guichet, A., & Bernard, F. (2021). Nuclear Migration in the Drosophila Oocyte.
JoVE (Journal of Visualized Experiments),
171, e62688.
https://doi.org/10.3791/62688
Bernard, F., Jouette, J., Durieu, C., Le Borgne, R., Guichet, A., & Claret, S. (2021). GFP-Tagged Protein Detection by Electron Microscopy Using a GBP-APEX Tool in Drosophila.
Frontiers in Cell and Developmental Biology,
9.
https://www.frontiersin.org/articles/10.3389/fcell.2021.719582
Mukherjee, A., Brooks, P. S., Bernard, F., Guichet, A., & Conduit, P. T. (2020). Microtubules originate asymmetrically at the somatic golgi and are guided via Kinesin2 to maintain polarity within neurons.
ELife,
9, e58943.
https://doi.org/10.7554/eLife.58943
Métivier, M., Monroy, B. Y., Gallaud, E., Caous, R., Pascal, A., Richard-Parpaillon, L., Guichet, A., Ori-McKenney, K. M., & Giet, R. (2019). Dual control of Kinesin-1 recruitment to microtubules by Ensconsin in Drosophila neuroblasts and oocytes.
Development (Cambridge, England),
146(8), dev171579.
https://doi.org/10.1242/dev.171579
Jouette, J., Guichet, A., & Claret, S. B. (2019). Dynein-mediated transport and membrane trafficking control PAR3 polarised distribution.
ELife,
8, e40212.
https://doi.org/10.7554/eLife.40212
Bernard, F., Lepesant, J.-A., & Guichet, A. (2018). Nucleus positioning within Drosophila egg chamber.
Seminars in Cell & Developmental Biology,
82, 25–33.
https://doi.org/10.1016/j.semcdb.2017.10.013
Tissot, N., Lepesant, J.-A., Bernard, F., Legent, K., Bosveld, F., Martin, C., Faklaris, O., Bellaïche, Y., Coppey, M., & Guichet, A. (2017). Distinct molecular cues ensure a robust microtubule-dependent nuclear positioning in the Drosophila oocyte.
Nature Communications,
8(1), 15168.
https://doi.org/10.1038/ncomms15168
White, P. M., Serbus, L. R., Debec, A., Codina, A., Bray, W., Guichet, A., Lokey, R. S., & Sullivan, W. (2017). Reliance of Wolbachia on High Rates of Host Proteolysis Revealed by a Genome-Wide RNAi Screen of Drosophila Cells.
Genetics,
205(4), 1473–1488.
https://doi.org/10.1534/genetics.116.198903
Jouette, J., Claret, S., & Guichet, A. (2017). Phosphoinositides and Cell Polarity in the Drosophila Egg Chamber. In M. Kloc (Ed.),
Oocytes: Maternal Information and Functions (pp. 169–187). Springer International Publishing.
https://doi.org/10.1007/978-3-319-60855-6_8
Debec, A., Megraw, T. L., & Guichet, A. (2016). Methods to Establish Drosophila Cell Lines. In C. Dahmann (Ed.),
Drosophila: Methods and Protocols (pp. 333–351). Springer.
https://doi.org/10.1007/978-1-4939-6371-3_21
Le Droguen, P.-M., Claret, S., Guichet, A., & Brodu, V. (2015). Microtubule-dependent apical restriction of recycling endosomes sustains adherens junctions during morphogenesis of the Drosophila tracheal system.
Development,
142(2), 363–374.
https://doi.org/10.1242/dev.113472
Legent, K., Tissot, N., & Guichet, A. (2015). Visualizing Microtubule Networks During Drosophila Oogenesis Using Fixed and Live Imaging. In D. P. Bratu & G. P. McNeil (Eds.),
Drosophila Oogenesis: Methods and Protocols (pp. 99–112). Springer.
https://doi.org/10.1007/978-1-4939-2851-4_7
Claret, S., Jouette, J., Benoit, B., Legent, K., & Guichet, A. (2014). PI(4,5)P2 Produced by the PI4P5K SKTL Controls Apical Size by Tethering PAR-3 in
Drosophila Epithelial Cells.
Current Biology,
24(10), 1071–1079.
https://doi.org/10.1016/j.cub.2014.03.056
Lecland, N., Debec, A., Delmas, A., Moutinho-Pereira, S., Malmanche, N., Bouissou, A., Dupré, C., Jourdan, A., Raynaud-Messina, B., Maiato, H., & Guichet, A. (2013). Establishment and mitotic characterization of new Drosophila acentriolar cell lines from DSas-4 mutant.
Biology Open,
2(3), 314–323.
https://doi.org/10.1242/bio.20133327
Baffet, A. D., Benoit, B., Januschke, J., Audo, J., Gourhand, V., Roth, S., & Guichet, A. (2012). Drosophila tubulin-binding cofactor B is required for microtubule network formation and for cell polarity.
Molecular Biology of the Cell,
23(18), 3591–3601.
https://doi.org/10.1091/mbc.e11-07-0633
Parrott, B. B., Chiang, Y., Hudson, A., Sarkar, A., Guichet, A., & Schulz, C. (2011). Nucleoporin98-96 Function Is Required for Transit Amplification Divisions in the Germ Line of Drosophila melanogaster.
PLOS ONE,
6(9), e25087.
https://doi.org/10.1371/journal.pone.0025087
Brodu, V., Baffet, A. D., Le Droguen, P.-M., Casanova, J., & Guichet, A. (2010). A Developmentally Regulated Two-Step Process Generates a Noncentrosomal Microtubule Network in
Drosophila Tracheal Cells.
Developmental Cell,
18(5), 790–801.
https://doi.org/10.1016/j.devcel.2010.03.015
Fabian, L., Wei, H.-C., Rollins, J., Noguchi, T., Blankenship, J. T., Bellamkonda, K., Polevoy, G., Gervais, L., Guichet, A., Fuller, M. T., & Brill, J. A. (2010). Phosphatidylinositol 4,5-bisphosphate Directs Spermatid Cell Polarity and Exocyst Localization in Drosophila.
Molecular Biology of the Cell,
21(9), 1546–1555.
https://doi.org/10.1091/mbc.e09-07-0582
Lachkar, S., Lebois, M., Steinmetz, M. O., Guichet, A., Lal, N., Curmi, P. A., Sobel, A., & Ozon, S. (2010). Drosophila Stathmins Bind Tubulin Heterodimers with High and Variable Stoichiometries.
The Journal of Biological Chemistry,
285(15), 11667–11680.
https://doi.org/10.1074/jbc.M109.096727
Compagnon, J., Gervais, L., Roman, M. S., Chamot-Bœuf, S., & Guichet, A. (2009). Interplay between Rab5 and PtdIns(4,5)P2 controls early endocytosis in the Drosophila germline.
Journal of Cell Science,
122(1), 25–35.
https://doi.org/10.1242/jcs.033027
Nicolas, E., Chenouard, N., Olivo-Marin, J.-C., & Guichet, A. (2009). A Dual Role for Actin and Microtubule Cytoskeleton in the Transport of Golgi Units from the Nurse Cells to the Oocyte Across Ring Canals.
Molecular Biology of the Cell,
20(1), 556–568.
https://doi.org/10.1091/mbc.e08-04-0360
Gervais, L., Claret, S., Januschke, J., Roth, S., & Guichet, A. (2008). PIP5K-dependent production of PIP2 sustains microtubule organization to establish polarized transport in the Drosophila oocyte.
Development,
135(23), 3829–3838.
https://doi.org/10.1242/dev.029009
Januschke, J., Nicolas, E., Compagnon, J., Formstecher, E., Goud, B., & Guichet, A. (2007). Rab6 and the secretory pathway affect oocyte polarity in Drosophila.
Development,
134(19), 3419–3425.
https://doi.org/10.1242/dev.008078
GUICHET, A. (2007). Mise en place de la polarité au sein de l’ovocyte de drosophile : (R)évolution du développement. Mise En Place de La Polarité Au Sein de l’ovocyte de Drosophile : (R)Évolution Du Développement, 281, 24–27.
Januschke, J., Gervais, L., Gillet, L., Keryer, G., Bornens, M., & Guichet, A. (2006). The centrosome-nucleus complex and microtubule organization in the Drosophila oocyte.
Development,
133(1), 129–139.
https://doi.org/10.1242/dev.02179
Januschke, J., Gervais, L., Dass, S., Kaltschmidt, J. A., Lopez-Schier, H., Johnston, D. St., Brand, A. H., Roth, S., & Guichet, A. (2002). Polar Transport in the
Drosophila Oocyte Requires Dynein and Kinesin I Cooperation.
Current Biology,
12(23), 1971–1981.
https://doi.org/10.1016/S0960-9822(02)01302-7
Tazuke, S. I., Schulz, C., Gilboa, L., Fogarty, M., Mahowald, A. P., Guichet, A., Ephrussi, A., Wood, C. G., Lehmann, R., & Fuller, M. T. (2002). A germline-specific gap junction protein required for survival of differentiating early germ cells.
Development,
129(10), 2529–2539.
https://doi.org/10.1242/dev.129.10.2529
Guichet, A., Wucherpfennig, T., Dudu, V., Etter, S., Wilsch‐Bräuniger, M., Hellwig, A., González‐Gaitán, M., Huttner, W. B., & Schmidt, A. A. (2002). Essential role of endophilin A in synaptic vesicle budding at the Drosophila neuromuscular junction.
The EMBO Journal,
21(7), 1661–1672.
https://doi.org/10.1093/emboj/21.7.1661
Ozon, S., Guichet, A., Gavet, O., Roth, S., & Sobel, A. (2002). Drosophila Stathmin: A Microtubule-destabilizing Factor Involved in Nervous System Formation.
Molecular Biology of the Cell,
13(2), 698–710.
https://doi.org/10.1091/mbc.01-07-0362
Guichet, A., Peri, F., & Roth, S. (2001). Stable Anterior Anchoring of the Oocyte Nucleus Is Required to Establish Dorsoventral Polarity of the
Drosophila Egg.
Developmental Biology,
237(1), 93–106.
https://doi.org/10.1006/dbio.2001.0354
Jiménez, G., Guichet, A., Ephrussi, A., & Casanova, J. (2000). Relief of gene repression by torso RTK signaling: role of capicua in Drosophila terminal and dorsoventral patterning. Genes & Development, 14(2), 224–231.
Tomancak, P., Guichet, A., Zavorszky, P., & Ephrussi, A. (1998). Oocyte polarity depends on regulation of gurken by Vasa.
Development,
125(9), 1723–1732.
https://doi.org/10.1242/dev.125.9.1723
Florence, B., Guichet, A., Ephrussi, A., & Laughon, A. (1997). Ftz-F1 is a cofactor in Ftz activation of the Drosophila engrailed gene.
Development,
124(4), 839–847.
https://doi.org/10.1242/dev.124.4.839
Guichet, A., Copeland, J. W. R., Erdélyi, M., Hlousek, D., Závorszky, P., Ho, J., Brown, S., Percival-Smith, A., Krause, H. M., & Ephrussi, A. (1997). The nuclear receptor homologue Ftz-F1 and the homeodomain protein Ftz are mutually dependent cofactors.
Nature,
385(6616), 548–552.
https://doi.org/10.1038/385548a0
Erdélyi, M., Michon, A.-M., Guichet, A., Glotzer, J. B., & Ephrussi, A. (1995). Requirement for Drosophila cytoplasmic tropomyosin in oskar mRNA localization.
Nature,
377(6549), 524–527.
https://doi.org/10.1038/377524a0
Overexpression of Partner of Numb Induces Asymmetric Distribution of the PI4P 5-Kinase Skittles in Mitotic Sensory Organ Precursor Cells in Drosophila | PLOS ONE. (n.d.). Retrieved February 19, 2024, from
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0003072
Overexpression of Partner of Numb Induces Asymmetric Distribution of the PI4P 5-Kinase Skittles in Mitotic Sensory Organ Precursor Cells in Drosophila | PLOS ONE. (n.d.). Retrieved February 19, 2024, from
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0003072
Thèses
- Maëlys Loh (2022) : Asymétries moléculaires contrôlant le positionnement du noyau dans l’ovocyte de drosophila melanogaster
- Julie Jouette (2017) : Phosphoinositides et contrôle de la polarité cellulaire : régulations croisées entre la PIP5K Skittles et les protéines de polarité PAR1 et PAR3.
- Nicolas Tissot (2015) : Relation croisée entre le positionnement du noyau et l’organisation des microtubules dans la polarisation de l’ovocyte chez la drosophile : approche par microscopie optique ex-vivo et photomanipulation
- Pierre-Marie Le Droguen (2013) : Rôle du réseau de microtubules lors de la morphogénèse du système trachéal dans l’embryon de drosophile
- Alexandre Baffet (2010) : Organisation des microtubules et polarité cellulaire chez la Drosophile
- Julien Compagnon (2008) : Etude du trafic vésiculaire au cours de l’ovogenèse chez la Drosophile
- Louis Gervais (2006): Etude des relations entre la dynamique du réseau de microtubules et le transport polarisé dans l’ovocyte.
- Jens Januschke (2005) : mRNA localization in the Drosophila oocyte
Collaborations
- Dr. Jordi Casanova, Institut de Ciencies de Materials de Barcelona (CSIC), Barcelona, Spain
- Pr. Alexander Ludwig, Nanyang Technological University, Singapore
- Dr. Vladimir Gelfand, Northwestern University, Chicago, USA
- Dr Régis Giet, Institut de Génétique et Développement de Rennes, Rennes
- Dr Paul Conduit, Institut Jacques Monod, Paris
Financement
- Nuclear Deformation in Eukaryotes, Projet Emergence en Recherche, IDEX Université Paris Cité (coordinateurs Fred Bernard et Sylvain Brun)
Actualités
Emploi
L’équipe Guichet recrute :
- Funded PhD Position (3 years, starting 09/2025) Microtubule organization and nuclear positioning : pdf offer
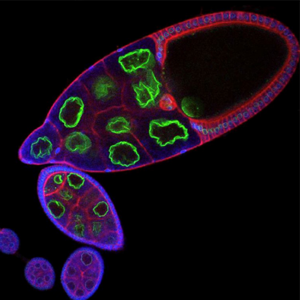


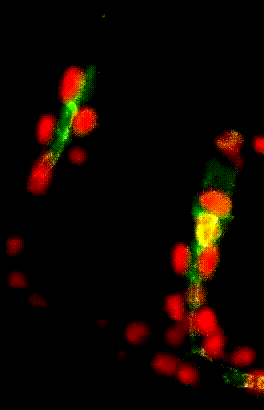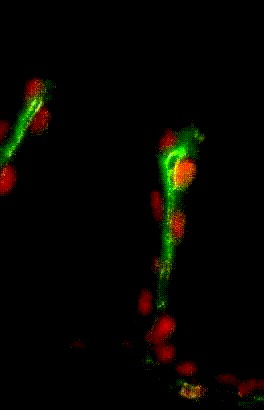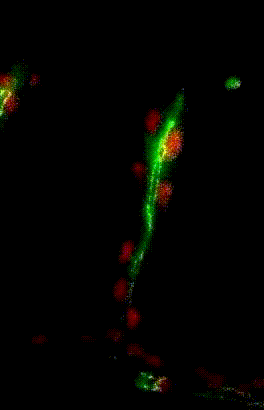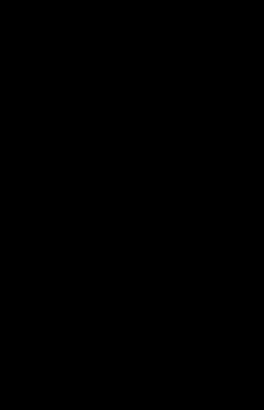
 Frédéric BERNARD, Assistant Professor, GUICHET LAB+33 (0)1 57 27 80 77, bureau 422B
Frédéric BERNARD, Assistant Professor, GUICHET LAB+33 (0)1 57 27 80 77, bureau 422B Veronique BRODU, Researcher, GUICHET LAB+33 (0)1 57 27 80 78, bureau 422B
Veronique BRODU, Researcher, GUICHET LAB+33 (0)1 57 27 80 78, bureau 422B Sylvain BRUN, Assistant Professor, GUICHET LAB+33 (0)1 57 27 80 87, bureau 422B
Sylvain BRUN, Assistant Professor, GUICHET LAB+33 (0)1 57 27 80 87, bureau 422B Sandra CARVALHO, PhD student, GUICHET LAB+33 (0)1 57 27 80 76, bureau 422B
Sandra CARVALHO, PhD student, GUICHET LAB+33 (0)1 57 27 80 76, bureau 422B Sandra CLARET, Assistant Professor, GUICHET LAB+33 (0)1 57 27 80 77, bureau 422B
Sandra CLARET, Assistant Professor, GUICHET LAB+33 (0)1 57 27 80 77, bureau 422B Zoe KACHANER, Intern, GUICHET LAB
Zoe KACHANER, Intern, GUICHET LAB Jean-Antoine LEPESANT, Emeritus researcher, GUICHET LAB+33 (0)1 57 27 80 78, bureau 422B
Jean-Antoine LEPESANT, Emeritus researcher, GUICHET LAB+33 (0)1 57 27 80 78, bureau 422B Maxime PONTHIER, Intern, GUICHET LAB
Maxime PONTHIER, Intern, GUICHET LAB Fanny ROLAND-GOSSELIN, PhD student, GUICHET LAB+33 (0)1 57 27 80 77, bureau 422B
Fanny ROLAND-GOSSELIN, PhD student, GUICHET LAB+33 (0)1 57 27 80 77, bureau 422B Wiam SINDABAD, Intern, GUICHET LAB
Wiam SINDABAD, Intern, GUICHET LAB

