Mécanotransduction : de la surface de la cellule à son noyau
NICOLAS BORGHI
Notre équipe étudie comment nos cellules réagissent à leur environnement mécanique. Nous proposons une approche par microscopies de fluorescence quantitatives et ingénierie génétique pour des réponses de la molécule au tissu.
Mots-clés : mécanotransduction, adhésion cellulaire, migration cellulaire, microscopie, biosenseurs, biophysique
+33 (0)1 57 27 80 41 Contact
Dans les organismes multicellulaires, les cellules génèrent et subissent des forces mécaniques qui se propagent dans tout l’organisme. Ces forces peuvent déterminer la forme des tissus et organes, et réguler des programmes génétiques. Les mécanismes moléculaires de la transmission des forces mécaniques et de leur transduction en signaux biochimiques sont cependant mal connus.
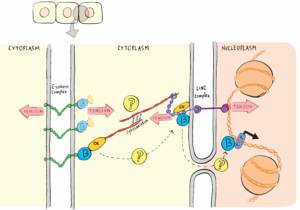
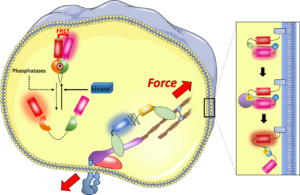
Notre projet porte sur les complexes macromoléculaires qui transmettent et transduisent ces signaux mécaniques à l’intérieur et entre les cellules, et les fonctions cellulaires qui en dépendent. Nous nous intéressons aux récepteurs d’adhésion de la membrane plasmique, aux complexes transmembranaires de l’enveloppe nucléaire et leurs fonctions dans l’adhésion cellulaire, la migration, la prolifération et l’activité transcriptionnelle.
Pour répondre à cet objectif, nous développons et utilisons des biosenseurs génétiquement encodés et des méthodes de microscopie et de micromanipulation avancées sur des systèmes modèles de culture cellulaire. Cette combinaison permet de contrôler et mesurer dynamiquement et quantitativement le comportement des complexes de protéines et des cellules dans un large éventail d’échelles de temps et de longueur.
Membres
 Nicolas AUDUGE, Light microscopy engineer, BORGHI LAB+33 (0)1 57 27 80 42, bureau 302B
Nicolas AUDUGE, Light microscopy engineer, BORGHI LAB+33 (0)1 57 27 80 42, bureau 302B Mariia BALATSKAIA, Postdoctoral researcher, BORGHI LAB+33 (0)1 57 27 80 41, bureau 302B
Mariia BALATSKAIA, Postdoctoral researcher, BORGHI LAB+33 (0)1 57 27 80 41, bureau 302B Jonas CLEMENCE, Intern, BORGHI LAB
Jonas CLEMENCE, Intern, BORGHI LAB Maïté COPPEY, Emeritus researcher, BORGHI LAB+33 (0)1 57 27 80 45, bureau 302B
Maïté COPPEY, Emeritus researcher, BORGHI LAB+33 (0)1 57 27 80 45, bureau 302B Thomas GERMIER, Postdoctoral researcher, BORGHI LAB+33 (0)1 57 27 80 41, bureau 302B
Thomas GERMIER, Postdoctoral researcher, BORGHI LAB+33 (0)1 57 27 80 41, bureau 302B Philippe GIRARD, Assistant Professor, BORGHI LAB+33 (0)1 57 27 80 42, bureau 302B
Philippe GIRARD, Assistant Professor, BORGHI LAB+33 (0)1 57 27 80 42, bureau 302B Hugo LACHUER, Postdoctoral researcher, BORGHI LAB+33 (0)1 57 27 80 41, bureau 302B
Hugo LACHUER, Postdoctoral researcher, BORGHI LAB+33 (0)1 57 27 80 41, bureau 302B
Pour contacter un membre de l’équipe par mail : prenom.nom@ijm.fr
1: Déjardin T, Carollo PS, Sipieter F, Davidson PM, Seiler C, Cuvelier D, Cadot, B, Sykes C, Gomes ER, Borghi N. Nesprins are mechanotransducers that discriminate epithelial-mesenchymal transition programs. J Cell Biol. 2020 Oct 5;219(10):e201908036. doi: 10.1083/jcb.201908036. PMID: 32790861; PMCID: PMC7659719.
2: Davidson PM, Battistella A, Déjardin T, Betz T, Plastino J, Borghi N, Cadot, B, Sykes C. Nesprin-2 accumulates at the front of the nucleus during confined. cell migration. EMBO Rep. 2020 Jul 3;21(7):e49910. doi: 10.15252/embr.201949910. Epub 2020 May 17. PMID: 32419336; PMCID: PMC7332974.
3: Audugé N, Padilla-Parra S, Tramier M, Borghi N, Coppey-Moisan M. Chromatin condensation fluctuations rather than steady-state predict chromatin accessibility. Nucleic Acids Res. 2019 Jul 9;47(12):6184-6194. doi: 10.1093/nar/gkz373. PMID: 31081027; PMCID: PMC6614833.
4: De Pascalis C, Pérez-González C, Seetharaman S, Boëda B, Vianay B, Burute M, Leduc C, Borghi N, Trepat X, Etienne-Manneville S. Intermediate filaments control collective migration by restricting traction forces and sustaining cell-cell contacts. J Cell Biol. 2018 Sep 3;217(9):3031-3044. doi: 10.1083/jcb.201801162. Epub 2018 Jul 6. PMID: 29980627; PMCID: PMC6122997.
5: Gayrard C, Bernaudin C, Déjardin T, Seiler C, Borghi N. Src- and confinement- dependent FAK activation causes E-cadherin relaxation and β-catenin activity. J Cell Biol. 2018 Mar 5;217(3):1063-1077. doi: 10.1083/jcb.201706013. Epub 2018 Jan 8. PMID: 29311227; PMCID: PMC5839785.
6: Sarangi BR, Gupta M, Doss BL, Tissot N, Lam F, Mège RM, Borghi N, Ladoux B. Coordination between Intra- and Extracellular Forces Regulates Focal Adhesion. Dynamics. Nano Lett. 2017 Jan 11;17(1):399-406. doi: 10.1021/acs.nanolett.6b04364. Epub 2016 Dec 23. PMID: 27990827; PMCID: PMC5423523.
7: Liu Z, Bun P, Audugé N, Coppey-Moisan M, Borghi N. Vinculin head-tail interaction defines multiple early mechanisms for cell substrate rigidity sensing. Integr Biol (Camb). 2016 Jun 13;8(6):693-703. doi: 10.1039/c5ib00307e. Epub 2016 May 11. PMID: 27169142.
8: Lowndes M, Rakshit S, Shafraz O, Borghi N, Harmon RM, Green KJ, Sivasankar S, Nelson WJ. Different roles of cadherins in the assembly and structural integrity of the desmosome complex. J Cell Sci. 2014 May 15;127(Pt 10):2339-50. doi: 10.1242/jcs.146316. Epub 2014 Mar 7. PMID: 24610950; PMCID: PMC4021477.
9: Borghi N, Sorokina M, Shcherbakova OG, Weis WI, Pruitt BL, Nelson WJ, and Alexander R. Dunn. E-cadherin is under constitutive actomyosin-generated tension that is increased at cell-cell contacts upon externally applied stretch. Proc Natl Acad Sci U S A. 2012 Jul 31;109(31):12568-73. doi: 10.1073/pnas.1204390109. Epub 2012 Jul 16. Erratum in: Proc Natl Acad Sci U S A. 2012 Nov 13;109(46):19034. PMID: 22802638; PMCID: PMC3411997.
10: Borghi N, Lowndes M, Maruthamuthu V, Gardel ML, Nelson WJ. Regulation of cell motile behavior by crosstalk between cadherin- and integrin-mediated adhesions. Proc Natl Acad Sci U S A. 2010 Jul 27;107(30):13324-9. doi: 10.1073/pnas.1002662107. Epub 2010 Jun 21. PMID: 20566866; PMCID: PMC2922157.
Publications
Revue
Chapitre de livre
2017: Charlène Gayrard – Mécanotransduction au complexe E-cadhérine/bêta-caténine lors de la transition épithélio-mésenchymateuse.
2019: Pietro Salvatore Carollo – The LINC complex is a mechanotransducer that regulates catenin signalling dyring epithelial-mesenchymal transitions.
2021: Helena Canever – Regulation of epithelial migration by cell adhesions: roles of vinculin.
Ongoing: Louis Laurent.
- Michel Labouesse – IBPS
- Benoît Ladoux / René-Marc Mège – IJM
- Jean-Baptiste Manneville – MSC
- Stefano Marullo – Institut Cochin
- Matthieu Piel – IPGG
- Vanessa Ribes – IJM
- Fondation ARC pour la recherche sur le cancer
- Agence Nationale de la Recherche (ANR)
- Labex Who Am I?
Check this preprint out of a great collaboration with the Gloerich and Trepat labs: https://doi.org/10.1101/2021.11.29.470352