Compartimentation intracellulaire
Ishier Raote
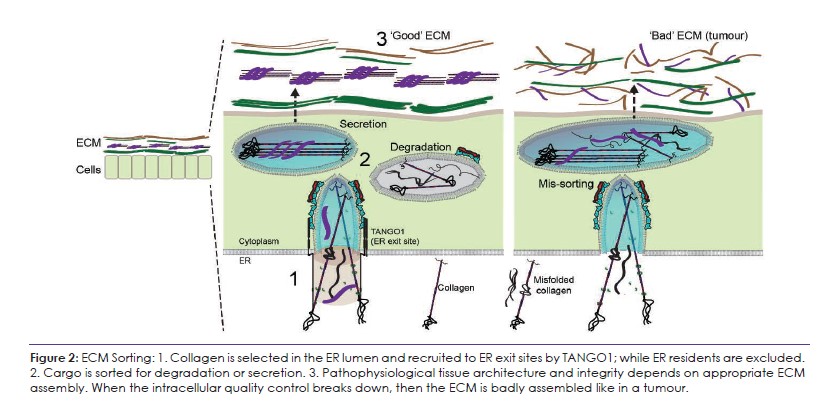
Tous les animaux possèdent une matrice extracellulaire (MEC) – des matériaux sécrétés qui s’assemblent en un échafaudage biomécanique en trois dimensions qui définit la forme des tissus multicellulaires. La MEC représente jusqu’à 70 % de notre poids sec et ses composants les plus abondants sont les collagènes, qui représentent à eux seuls 25 % du poids des protéines de l’organisme. Pour un assemblage correct de la MEC, les cellules doivent sécréter des collagènes pliés et dégrader les collagènes mal pliés. Lorsque la sécrétion ou la dégradation ne se fait pas correctement, la MEC est mal assemblée. La manière dont les cellules contrôlent la quantité et la qualité des collagènes sécrétés et des autres protéines de la MEC n’est pas encore claire. L’équipe Raote vise à révéler les mécanismes fondamentaux du tri du collagène dans la voie sécrétoire précoce et comment les erreurs dans ce contrôle conduisent à des changements pathophysiologiques.
Mots clés : matrice extracellulaire, collagène
Tous les animaux possèdent une matrice extracellulaire (MEC) dont les composants les plus abondants sont les collagènes, qui représentent à eux seuls 25 % du poids des protéines de l’organisme. Pour que l’assemblage de la MEC soit correct, les cellules doivent sécréter des collagènes pliés et dégrader les collagènes en excès ou mal pliés. Lorsque la sécrétion ou la dégradation se fait mal, la MEC résultante est mal assemblée, ce qui entraîne des problèmes dans la construction des tissus et la formation de structures aberrantes telles que les tumeurs. Les mécanismes cellulaires de ce tri des collagènes intracellulaires en vue de leur sécrétion ou de leur dégradation dans la voie sécrétoire restent inconnus.
Les collagènes constituent une cargaison sécrétoire difficile pour les cellules car ils sont énormes. Mes découvertes de TANGO1 en tant qu’organisateur principal de l’interface ERES-Golgi ont permis de comprendre comment les cargaisons volumineuses et de grand volume comme le collagène sont sécrétées. Ces études constituent une plate-forme idéale pour révéler les mécanismes fondamentaux du tri du collagène entre la sécrétion et la dégradation, et la manière dont le processus de tri contribue à la construction des tissus.
La voie sécrétoire précoce, à l’interface entre les sites de sortie du réticulum endoplasmique (ERES), le compartiment intermédiaire ER-Golgi (ERGIC) et le Golgi, est une passerelle vitale pour contrôler la quantité et la qualité des collagènes, qui s’assemblent ensuite en une MEC. Mon groupe vise à révéler les mécanismes par lesquels TANGO1 et sa machinerie ERES associée agissent comme un point de contrôle de la qualité et de la quantité du collagène, ce qui permettra de mieux comprendre les conditions physiopathologiques associées à l’altération de l’assemblage de la MEC et des tissus, y compris la tumorigenèse (Figure 2). Pour commencer, nous continuerons à utiliser le collagène comme modèle, puis nous inclurons d’autres grandes protéines de la MEC, notamment la laminine, la fibrilline 1 et la fibronectine. Nos études sur les étapes intracellulaires de la sécrétion du collagène :
1. Reconstituer la « filtration » du collagène au niveau de l’ERES, afin de comprendre comment les collagènes dépliés (prématurés) sont empêchés de sortir de l’ERES.
Lorsque le collagène se replie, il forme une structure rigide en forme de tige à triple hélice. Cette stabilité et cette rigidité sont essentielles à l’assemblage correct de la MEC. Le collagène non replié (prématuré) ne doit pas être autorisé à quitter le RE et à être sécrété. On constate que les protéines prématurées sont retenues à l’ERES et empêchées de s’échapper, mais le mécanisme de cette rétention active dans le RE reste inconnu. Nos données préliminaires suggèrent que TANGO1 joue un rôle dans ce processus et en particulier ses régions intrinsèquement désordonnées (IDR) étendues dans la lumière du RE. Notre objectif est d’étudier comment les IDR contribuent à cette sorte de » filtration » du collagène dans la lumière du RE.
Une hypothèse suggère que les IDR de TANGO1 subissent une séparation de phase liquide-liquide, formant un sous-compartiment spécialisé dans la lumière du RE, qui sépare le collagène plié du collagène prématuré et d’autres résidents du RE qui ne devraient pas s’échapper du RE.
Notre groupe utilisera une approche ascendante avec des nanopores d’origami d’ADN pour reconstituer la dynamique et l’état physique des IDR qui tapissent le pore dans différentes conditions. Dans une approche descendante, nous produirons des mutations ciblées dans TANGO1 et dans la machinerie ERES connexe afin d’identifier d’autres composants du filtre et la manière dont ils fonctionnent ensemble.
2. Tri des collagènes mal repliés en phase terminale en vue de leur dégradation au niveau d’une ERES
Un problème inhabituel qui se pose lors du repliement du collagène est que parfois, au lieu de former une triple hélice rigide en forme de tige correctement repliée, il y a des interruptions dans la tige causées par des erreurs locales de repliement. Ces collagènes mal repliés ressemblent alors à une tige avec une « charnière ». Ces collagènes mal repliés ne peuvent pas être sécrétés et doivent être dégradés, mais les mécanismes de leur dégradation sont mal compris.
Des rapports récents montrent que l’ERES n’est pas seulement un site d’exportation des cargaisons sécrétoires, mais aussi un site de dégradation des collagènes excédentaires ou mal repliés en phase terminale, en les acheminant vers les lysosomes au lieu de les diriger vers le Golgi. On ne comprend pratiquement pas comment la machinerie ERES est utilisée dans ce tri. Nous identifierons cette machinerie moléculaire et la manière dont elle est réorientée pour les deux voies de trafic sécrétoire ou dégradatif. Nous introduirons des étiquettes « BioID » à l’extrémité cytoplasmique de TANGO1 dans les fibroblastes et les kératinocytes (cellules CCL1106) afin de biotinyler les protéines à proximité de l’ERES dans des conditions de sécrétion ou de dégradation. Les protéines biotinylées seront immunoprécipitées et identifiées par spectrométrie de masse. Les gènes cibles seront éliminés à l’aide de CRISPR/Cas9 pour tester leur rôle dans le contrôle de la qualité et de la quantité de collagène dans les fibroblastes et les kératinocytes.
3. Altérations physiopathologiques dans les tumeurs cutanées qui surviennent lorsque le tri ERES échoue. Avec le collaborateur Dr Neil Rajan (Newcastle, UK)
Nous collaborons avec le Dr Neil Rajan, dermatogénéticien clinique, expert en maladies génétiques rares de la peau. Ensemble, nous avons identifié une maladie rare, le syndrome cutané CYLD (CCS), avec de grandes tumeurs cutanées, où la machinerie ERES (en particulier TANGO1) est dégradée. Puisque TANGO1 est nécessaire à la sécrétion de collagène, on pourrait imaginer que les tumeurs sécrètent moins de collagène – mais de manière contre-intuitive, les tumeurs hypersécrètent du collagène ! Nous avons découvert que le collagène sécrété est souvent mal replié, même s’il n’aurait jamais dû quitter le RE, ce qui renforce le rôle de TANGO1 et d’autres machines ERES en tant que point de contrôle de la qualité et de la quantité. Nous étudierons le mauvais assemblage de la MEC dans ces tumeurs cutanées, en nous concentrant sur les effets de l’altération du contrôle de la qualité sur la MEC. Des cellules tumorales dérivées de patients atteints de SCC seront utilisées pour caractériser les différences dans l’ERES des cellules tumorales et identifier comment le point de contrôle de l’ERES est perdu dans les tumeurs. L’accent sera mis sur la révélation des mécanismes par lesquels les tumeurs dégradent TANGO1.
Dans l’ensemble, cette recherche sur la filtration, le tri et la dégradation du collagène a des implications importantes pour la compréhension et le traitement de la tumorigenèse ou des troubles liés à l’hypersécrétion d’ECM comme la fibrose, qui contribuent à environ 45 % de tous les décès dans les pays industrialisés.
Pour contacter un membre de l’équipe par mail : prenom.nom@ijm.fr

Publications
Preprint
Revue
Fondation ARC, FRM




