Polarité et morphogenèse
Antoine GUICHET
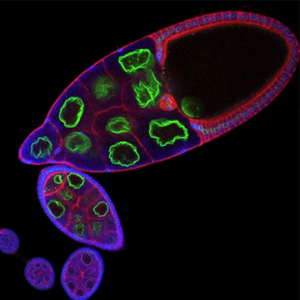
Notre équipe de recherche vise à élucider les mécanismes qui contrôlent la polarité cellulaire et la morphogenèse tissulaire en relation avec le cytosquelette et en particulier avec le réseau de microtubules.
Mots-clés : Polarité cellulaire, morphogénèse, microtubules, transport asymétrique, phospholipides, PAR complexes
+33 (0)157278076 antoine.guichet(at)ijm.fr @GuichetLab
La compréhension des mécanismes qui orchestrent la formation de tissus et d’organes et qui contrôlent le maintien de leur architecture est une question fondamentale en biologie. La formation des tissus et leur homéostasie sont coordonnées par des processus cellulaires incluant la polarité cellulaire, l’adhérence et la motilité. La compréhension de ces processus est aussi essentielle pour mieux comprendre le développement de pathologies comme le cancer.
Notre équipe de recherche vise à élucider les mécanismes qui contrôlent la polarité cellulaire et la morphogenèse tissulaire en relation avec le cytosquelette et en particulier avec le réseau de microtubules. Pour cela nous utilisons le développement de la drosophile comme modèle d’étude et nous concentrons notre recherche sur deux axes.
- Au niveau cellulaire, en étudiant le rôle du cytosquelette dans la mise en place de la polarité de l’ovocyte au cours de l’ovogenèse. Nous cherchons à déterminer les mécanismes impliqués dans le transport asymétrique de protéines et d’ARNs et ceux impliqués dans le positionnement asymétrique du noyau.
- Au niveau tissulaire, en étudiant l’implication du cytosquelette dans la morphogenèse tissulaire. Nous cherchons à identifier les mécanismes contrôlant la migration collective de cellules, assurant la formation du système respiratoire au cours de l’embryogénèse.
Les approches expérimentales utilisées au laboratoire combinent la génétique, la biophysique et des techniques de biologie cellulaire. De plus, la microscopie photonique de pointe sur tissus vivants ainsi que la microscopie électronique constituent le cœur de nos expériences quotidiennes.
Migration du noyau dans l’ovocyte de drosophile
Organisation des membranes dans l’ovocyte de drosophile
Migration collectives des cellules trachéales dans l’embryon de drosophile
Responsable :
Antoine GUICHET
Téléphone : +33 (0)157278076, +33 (0)157278087
Email : antoine.guichet(at)ijm.fr
Membres de l’équipe :
| Frédéric | BERNARD | Enseignant-chercheur |
| Véronique | BRODU | Chercheur |
| Sylvain | BRUN | Enseignant-chercheur |
| Sandra | CLARET | Enseignant-chercheur |
| Jean-Antoine | LEPESANT | Chercheur émérite |
| Sandra | CARVALHO | Doctorante |
| Fanny | ROLAND GOSSELIN | Doctorante |
| Joanna | AOUAD | Master 2 |
| Nicolas | ROBERT | Master 2 |
| Marie Caroline | VIRON | Master 2 |
The Importance of the Position of the Nucleus in Drosophila Oocyte Development. Lepesant JA, Roland-Gosselin F, Guillemet C, Bernard F, Guichet A. Cells. (2024)
Kinesin-1 promotes centrosome clustering and nuclear migration in the Drosophila oocyte. Development. Loh, M., Bernard, F., Guichet, A. (2023).
Dynein-mediated transport and membrane trafficking control PAR3 polarised distribution. Jouette J, Guichet A, Claret S. eLIFE (2019)
Distinct molecular cues ensure a robust microtubule-dependent nuclear positioning in the Drosophila oocyte.Tissot N, Lepesant JA, Bernard F, Legent K, Bosveld F, Martin C, Faklaris O, Bellaïche Y, Coppey M, Guichet A. Nature Communication. (2017)
Microtubule-dependent apical restriction of recycling endosomes sustains adherens junctions during morphogenesis of the Drosophila tracheal system. Le Droguen PM, Claret S, Guichet A, Brodu V. Development. (2015)
PI(4,5)P2 produced by the PI4P5K Skittles controls the apical domain size by tethering PAR-3 in Drosophila epithelial cells. Claret S, Benoit B, Richard-Ferrec G, Guichet A, Current Biology. (2014)
A developmentally regulated two-step process generates a non-centrosomal microtubule network. Brodu V, Baffet A, Le Droguen PM, Casanova J, Guichet A. Developmental Cell . (2010)
PIP5K-dependent production of PIP2 sustains microtubule organization to establish polarized transport in the Drosophila oocyte. Gervais L, Claret S, Januschke J, Roth S, Guichet A. Development (2008).
The Centrosome Nucleus complex directs the formation of two orthogonal microtubule polarized transport in the Drosophila oocyte Januschke J, Gervais L, Gillet, L., Keryer G, Bornen M, Guichet A. Development. (2006).
Polar transport in the Drosophila oocyte requires Dynein and Kinesin I cooperation, Januschke J, Gervais L, Dass S, Kaltschmidt J, Lopez-Schier H, St. Johnston D, Brand A, Roth S and Guichet A. Current Biology (2002).
Publications
- Maëlys Loh (2022) : Asymétries moléculaires contrôlant le positionnement du noyau dans l’ovocyte de drosophila melanogaster
- Julie Jouette (2017) : Phosphoinositides et contrôle de la polarité cellulaire : régulations croisées entre la PIP5K Skittles et les protéines de polarité PAR1 et PAR3.
- Nicolas Tissot (2015) : Relation croisée entre le positionnement du noyau et l’organisation des microtubules dans la polarisation de l’ovocyte chez la drosophile : approche par microscopie optique ex-vivo et photomanipulation
- Pierre-Marie Le Droguen (2013) : Rôle du réseau de microtubules lors de la morphogénèse du système trachéal dans l’embryon de drosophile
- Alexandre Baffet (2010) : Organisation des microtubules et polarité cellulaire chez la Drosophile
- Julien Compagnon (2008) : Etude du trafic vésiculaire au cours de l’ovogenèse chez la Drosophile
- Louis Gervais (2006): Etude des relations entre la dynamique du réseau de microtubules et le transport polarisé dans l’ovocyte.
- Jens Januschke (2005) : mRNA localization in the Drosophila oocyte
- Dr. Jordi Casanova, Institut de Ciencies de Materials de Barcelona (CSIC), Barcelona, Spain
- Pr. Alexander Ludwig, Nanyang Technological University, Singapore
- Dr. Vladimir Gelfand, Northwestern University, Chicago, USA
- Dr Régis Giet, Institut de Génétique et Développement de Rennes, Rennes
- Dr Paul Conduit, Institut Jacques Monod, Paris
Nuclear Deformation in Eukaryotes, Projet Emergence en Recherche, IDEX Université Paris Cité (coordinateurs Fred Bernard et Sylvain Brun).