Regulation of microtubule nucleation
Paul CONDUIT
Nous voulons acquérir une compréhension fondamentale de la manière dont la nucléation des microtubules est régulée spatio-temporellement dans le contexte des animaux pluricellulaires.
Nous sommes particulièrement intéressés par la façon dont les mécanismes régulant la formation et l’organisation des microtubules varient entre les types de cellules, y compris dans les cellules en division et les neurones.
Mots-clés : Microtubules, division cellulaire, neurones, gamma-tubuline, g-TuRC, MTOC, centrosome, drosophile
+33 (0)157278095 paul.conduit(at)ijm.fr @PaulConduit
Research goal
We want to gain a fundamental understanding of how microtubule nucleation is spatiotemporally regulated within the context of multi-cellular animals. We are particularly interested in how the mechanisms regulating microtubule formation and organisation vary between cell types.
Background
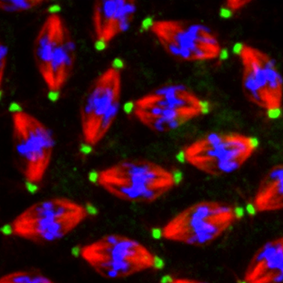
Microtubules are dynamic polymers that make up part of the cell’s cytoskeleton. They form a spectacular variety of arrays across different cell types and developmental stages. For example, during cell division microtubules are arranged into the mitotic spindle, which separates the duplicated chromosomes equally between daughter cells. In mature neurons, however, microtubules are arranged into polarised networks that run through axons and dendrites. These networks are required for structural support, neurite growth, and transport of molecules between the cell body and the neurite terminals. All cells use the same fundamental machinery to generate and organise microtubules, so how do different cells form such different microtubule arrays?
Research programme
We are addressing this question by studying the molecular regulation of multi-protein γ-tubulin ring complexes (γ-TuRCs), which template and catalyse de novo microtubule formation. γ-TuRCs are recruited and activated at specific sites within the cells in order to generate new microtubules at the right place and time. These sites include microtubule organising centres (MTOCs), such as centrosomes during mitosis or the Golgi in migrating fibroblasts, the sides of pre-existing microtubules, or specialised regions of cytoplasm, such as the cytosol surrounding mitotic chromatin. Once generated, molecular motors can slide microtubules against one another or even guide the direction of microtubule growth. Microtubules can also be stabilised by post-translational modifications or binding of other proteins. While not a focus of the lab, we are also interested in these post-nucleated processes as collectively they are important for correct microtubule array formation.
Main research areas currently within the lab
- Investigating the molecular mechanisms regulating γ-TuRC recruitment and activation at different MTOCs
- Understanding how microtubule formation and polarity are regulated within neurons.
Methodology
We predominantly use Drosophila as an in vivo multi-cellular animal model system. We combine precise manipulation of the genome, fixed and live advanced cell imaging, and biochemical assays to probe the molecular regulation of microtubules and the effect of their mis-regulation within cells.
Impact
Our work has implications for cancer and neurodegenerative disease, as γ-TuRCs have been identified as potential anti-cancer targets and microtubules form part of an important response during neuronal stress.
Group Leader :
Paul CONDUIT
phone : +33 (0)157278089
paul.conduit(at)ijm.fr
Members:
| Isabelle | BECAM | Assistant professor |
| Adria | CHORRO | PhD Student |
| Abir | ELFARKOUCHI | PhD Student |
| Léa | MAMMRI | Technician |
| Akila | MERAH | Postdoc |
| Anaëlle | TAIEB | Engineer |
| Berta | GARCIA | Intern |
Publications
Review
Preprint
Antoine Guichet, Susan Lea, Emmanuel Derivery, Françoise Ochsenbein
Impulscience, Fondation Bettencourt-Schueller
FRM – team label
ANR project grant
IDex, Université Parix Cité
21.11.2023 : Impulscience prize 2023
We are currently recruiting postdocs to study microtubule nucleation in neurons – please contact Paul Conduit directly.